
As filed with the Securities and Exchange Commission on August 11, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-3
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
ACTINIUM PHARMACEUTICALS,
INC.
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware |
74-2963609 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
100 Park Ave., 23rd Floor
New York, NY 10017
(646) 677-3870
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Sandesh Seth
Chairman and Chief Executive Officer
Actinium Pharmaceuticals, Inc.
100 Park Ave., 23rd Floor
New York, NY 10017
(646) 677-3870
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With Copies to:
Rick A. Werner, Esq.
Jayun Koo, Esq.
Haynes and Boone, LLP
30 Rockefeller Plaza, 26th Floor
New York, New York 10112
Tel. (212) 659-7300
Fax (212) 884-8234
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement, as determined by market conditions and other factors.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933, AS AMENDED, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY DETERMINE.
Explanatory Note
This Registration Statement contains two prospectuses:
| ● | a base prospectus which covers the offering, issuance and sale by us of up to $500,000,000 of our common stock, preferred stock, warrants, units and/or subscription rights; and |
| ● | a sales agreement prospectus covering the offering, issuance and sale by us of up to a maximum aggregate offering price of $200,000,000 of our common stock that may be issued and sold under the Amended and Restated Capital on Demand™ Sales Agreement, dated June 28, 2022, with JonesTrading Institutional Services LLC and B. Riley Securities, Inc. |
The base prospectus immediately follows this explanatory note. The specific terms of any securities to be offered pursuant to the base prospectus other than the shares under the sales agreement will be specified in a prospectus supplement to the base prospectus. The specific terms of the securities to be issued and sold under the sales agreement are specified in the sales agreement prospectus that immediately follows the base prospectus. The $200,000,000 of common stock that may be offered, issued and sold under the sales agreement prospectus is included in the $500,000,000 of securities that may be offered, issued and sold by us under the base prospectus. Upon termination of the sales agreement, any portion of the $200,000,000 included in the sales agreement prospectus that is not sold pursuant to the sales agreement will be available for sale in other offerings pursuant to the base prospectus and a corresponding prospectus supplement, and if no shares are sold under the sales agreement, the full $500,000,000 of securities may be sold in other offerings pursuant to the base prospectus and a corresponding prospectus supplement.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission, of which this prospectus is a part, is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offeror sale is not permitted
SUBJECT TO COMPLETION, DATED AUGUST 11, 2023
Prospectus

Actinium Pharmaceuticals, Inc.
$500,000,000
Common Stock
Preferred Stock
Warrants
Units
Subscription Rights
We may offer and sell from time to time, in one or more series or issuances and on terms that we will determine at the time of the offering, any combination of the securities described in this prospectus, up to an aggregate amount of $500,000,000.
We will provide specific terms of any offering in a supplement to this prospectus. Any prospectus supplement may also add, update, or change information contained in this prospectus. You should carefully read this prospectus and the applicable prospectus supplement as well as the documents incorporated or deemed to be incorporated by reference in this prospectus before you purchase any of the securities offered hereby.
These securities may be offered and sold in the same offering or in separate offerings to or through underwriters, dealers, and agents or directly to purchasers. The names of any underwriters, dealers, or agents involved in the sale of our securities, their compensation and any over-allotment options held by them will be described in the applicable prospectus supplement. See “Plan of Distribution.”
Our common stock is listed on the NYSE American under the symbol “ATNM.” On August 10, 2023, the last reported sale price of our common stock was $6.58 per share. We recommend that you obtain current market quotations for our common stock prior to making an investment decision. We will provide information in any applicable prospectus supplement regarding any listing of securities other than shares of our common stock on any securities exchange.
You should carefully read this prospectus, any prospectus supplement relating to any specific offering of securities, and all information incorporated by reference herein and therein.
Investing in our securities involves a high degree of risk. These risks are discussed in this prospectus under “Risk Factors” beginning on page 5 and in the documents incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2023.
Table of Contents
i
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission using a “shelf” registration process. Under this shelf process, we may, from time to time, sell any combination of the securities described in this prospectus in one or more offerings up to a total amount of $500,000,000. The offer and sale of securities under this prospectus may be made from time to time, in one or more offerings, in any manner described under the section in this prospectus entitled “Plan of Distribution.”
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add to, update or change information contained in the prospectus and, accordingly, to the extent inconsistent, information in this prospectus is superseded by the information in the prospectus supplement.
On June 28, 2022, we entered into an Amended and Restated Capital on Demand™ Sales Agreement, or the sales agreement, with JonesTrading and B. Riley Securities. A new prospectus with respect to the sales agreement is included in this registration statement, pursuant to which, in accordance with the terms of the sales agreement, we may, from time to time, offer and sell shares of our common stock having an aggregate offering price of up to $200,000,000.
The prospectus supplement to be attached to the front of this prospectus may describe, as applicable: the terms of the securities offered; the public offering price; the price paid for the securities; net proceeds; and the other specific terms related to the offering of the securities.
Any prospectus supplement may also add to, update or change information contained in the prospectus and, accordingly, to the extent inconsistent, information in this prospectus is superseded by the information in the prospectus supplement.
You should only rely on the information contained or incorporated by reference in this prospectus and any prospectus supplement or issuer free writing prospectus relating to a particular offering. No person has been authorized to give any information or make any representations in connection with this offering other than those contained or incorporated by reference in this prospectus, any accompanying prospectus supplement and any related issuer free writing prospectus in connection with the offering described herein and therein, and, if given or made, such information or representations must not be relied upon as having been authorized by us. Neither this prospectus nor any prospectus supplement nor any related issuer free writing prospectus shall constitute an offer to sell or a solicitation of an offer to buy offered securities in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. This prospectus does not contain all of the information included in the registration statement. For a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits.
You should read the entire prospectus and any prospectus supplement and any related issuer free writing prospectus, as well as the documents incorporated by reference into this prospectus or any prospectus supplement or any related issuer free writing prospectus, before making an investment decision. Neither the delivery of this prospectus or any prospectus supplement or any issuer free writing prospectus nor any sale made hereunder shall under any circumstances imply that the information contained or incorporated by reference herein or in any prospectus supplement or issuer free writing prospectus is correct as of any date subsequent to the date hereof or of such prospectus supplement or issuer free writing prospectus, as applicable. You should assume that the information appearing in this prospectus, any prospectus supplement or any document incorporated by reference is accurate only as of the date of the applicable documents, regardless of the time of delivery of this prospectus or any sale of securities. Our business, financial condition, results of operations and prospects may have changed since that date.
ii
This summary provides an overview of selected information contained elsewhere or incorporated by reference in this prospectus and does not contain all of the information you should consider before investing in our securities. You should carefully read the prospectus, any prospectus supplement, the information incorporated by reference and the registration statement of which this prospectus is a part in their entirety before investing in our securities, including the information discussed under “Risk Factors” in this prospectus, any prospectus supplement, and the documents incorporated by reference and our financial statements and notes thereto that are incorporated by reference in this prospectus. As used in this prospectus, unless the context otherwise indicates, the terms “we,” “our,” “us,” or “the Company” refer to Actinium Pharmaceuticals, Inc., a Delaware corporation, and its subsidiaries taken as a whole.
The Company
Actinium Pharmaceuticals, Inc. (“Actinium”) develops targeted radiotherapies intended to meaningfully improve survival for patients with relapsed or refractory cancer who have failed existing therapies. Our vision is to build a specialty, hospital-focused, radiotherapeutics company that develops and markets medicines for patients who are treated primarily in large quaternary care hospitals and their catchment areas.
We intend to leverage the clinical data of our lead product candidates, Iomab-B and Actimab-A, to improve outcomes in patients with relapsed or refractory acute myeloid leukemia (“r/r AML”) by launching two radiotherapy drugs over the next several years to address the significant need for better outcomes from treatment with therapeutics or from undergoing a bone marrow transplant (“BMT”).
We also intend to further advance Iomab-B outside of acute myeloid leukemia (“AML”) based on promising data as a disease control and conditioning agent for various other blood cancers. Based on early promising clinical trial results, we are also working on a lower dose, next generation conditioning program, Iomab-ACT, for rapidly growing cell and gene therapies.
Our Clinical Pipeline
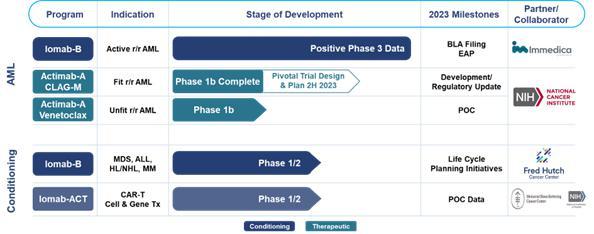
AML is an aggressive, heterogeneous disease that is difficult-to-treat. Most AML patients develop relapsed or refractory disease within one year of being afflicted and have an extremely poor prognosis and dismal survival. Currently, a BMT is the only curative regimen available for AML patients, however, access is limited to AML patients who are fit enough to withstand the challenges associated with this treatment. The majority of AML patients are considered not transplantable in routine clinical practice as they are not fit enough to withstand the rigors of the patient journey which includes therapy to attain a remission, conditioning regimens to destroy diseased marrow, challenge of the transplant itself or post-transplant complications.
Our Iomab-B and Actimab-A product candidates potentially fill the major unmet medical needs in r/r AML in a complementary fashion as they are directed at different parts of the patient journey. Iomab-B is being developed as a targeted bridging therapy candidate that we believe could provide both disease control and conditioning in one agent. We believe results from our Phase 3 SIERRA trial demonstrate the possibility for unprecedented access to a BMT and improved survival in unfit patients who are currently not considered transplantable in routine clinical practice. We are developing Actimab-A as a targeted therapy candidate for fit patients. Actimab-A has demonstrated an extension in survival in a proof-of-concept study and is poised for advanced development in collaboration with the NCI, or National Cancer Institute (“NCI”). Together, we believe these two product candidates could provide us the opportunity to transform the treatment of AML, especially in the relapsed and refractory segment which represents over 50% of AML patients.
1
We announced in October 2022 that Iomab-B met the primary endpoint of durable Complete Remission (“dCR”) with a high degree of statistical significance (p<0.0001) in the pivotal Phase 3 Study of Iomab-B in Elderly Relapsed or Refractor AML, or “SIERRA trial.” In February 2023, we announced full trial results, demonstrating unprecedented transplant access and improved outcomes in patients with r/r AML, with double 1-year and median overall survival (“OS”) compared to control arm patients. These data were presented at the 2023 Tandem Meetings aka the Transplantation & Cellular Therapy (“TCT”) Meetings of the American Society for Transplantation and Cellular Therapy (“ASTCT”) and the Center for International Blood & Marrow Transplant Research (“CIBMTR”). We believe these results from the SIERRA trial may provide the opportunity, if we are able to obtain U.S. Food and Drug Administration (“FDA”) approval, to establish Iomab-B as a new standard of care.
The results from the SIERRA trial were recently presented and discussed at major bone marrow transplant and hematology medical conferences, nuclear medicines conference and nursing congress, which is helping to broaden the awareness of Iomab-B among members of the relevant medical and scientific communities as we prepare for potential commercialization if we achieve FDA approval. Including TCT, the SIERRA Phase 3 results have now been highlighted in oral presentations at four international medical conferences in the U.S. and EU attended by key Iomab-B stakeholders including bone marrow transplant physicians, hematologists and nuclear medicine physicians. On April 27, 2023, the results from the SIERRA trial were also showcased at the European Society for Blood and Marrow Transplantation (“EBMT”) 49th Annual Meeting, which was well-attended by the European transplant community. Also in April 2023, two posters that detailed clinical findings from the SIERRA trial sites were presented at the 48th Annual Oncology Nursing Society (“ONS”) Congress. We believe that the scientific community present at such events took note of the successful administration of Iomab-B infusions at various BMT centers, which was done without increasing radiation exposure risks to treating nursing staff. SIERRA results were also awarded an oral presentation at the European Hematology Association (“EHA”) 2023 Hybrid Congress and presented in Frankfurt, Germany on June 10, 2023. The SIERRA results were also presented at the Society for Nuclear Medicine and Molecule Imaging (“SNMMI”) annual meeting on June 26, 2023 and was awarded the Henry N. Wagner, Jr., Abstract of the Year award, which represents the top selection out of more than 1,500 abstracts accepted for presentation.
We are actively working on launching an early access program (“EAP”) for Iomab-B. We are also working towards completing and submitting our Biologics License Application (“BLA”) for Iomab-B to the FDA by the end of 2023 and if approved, we intend to commercialize Iomab-B in the U.S. We are committed to bringing Iomab-B to patients globally and are working with Immedica AB (“Immedica”), our European, Middle East and North Africa (“EUMENA”) partner, for the subsequent marketing authorization application (“MAA”) of Iomab-B with the European Medicines Agency (“EMA”). Europe represents a large commercial market opportunity with approximately twice as many transplants performed in Europe compared to the U.S.
Actimab-A is being developed under what we believe to be the current industry-leading clinical-study program utilizing the potent alpha radiation emitting isotope Actinium-225 (“Ac-225”) with clinical data in approximately 150 patients treated over six clinical trials. The potent linear energy transfer emitted by Ac-225 has no known resistance mechanism. Actimab-A is being developed in combination with other regimens to exploit mechanistic synergies and leverage the mutation-agnostic mechanism of action of Ac-225 with the objective of establishing it as a backbone therapy in AML, an extremely heterogenous disease.
We believe our Actimab-A + CLAG-M therapeutic combination trial results in r/r AML provide validation of this approach. Such results were presented at the American Society of Hematology (“ASH”) 2022 Annual Meeting & Exposition in December 2022. Phase 1 results from the Actimab-A + CLAG-M combination trial showed high response rates and minimal residual disease (“MRD”) negativity, translating to a survival benefit of 53% and 32% at one and two years in patients who are typically expected to live two to four months. At the same meeting, we shared Phase 1 data showing that the combination of Actimab-A + venetoclax was well-tolerated with responses, including a Complete Remission (“CR”) and a partial response in early dose escalation cohorts. We believe the promise of these results have paved the way for the NCI Cooperative Research and Development Agreement (“CRADA”), announced on February 6, 2023, to develop Actimab-A for the treatment of patients with AML and other hematologic malignancies. Under the CRADA, Actinium expects to initiate the late-stage development of Actimab-A, including a potential pivotal trial, in combination as a backbone therapy for r/r AML and expects to update on the program and timing by end of the second half of 2023.
2
To explore the potential for a broader development opportunity with our Actimab-A program, we are studying the potential use of Actimab-A in solid tumor indications through our R&D efforts. CD33-expressing myeloid derived suppressor cells (“MDSCs”) are present within the tumor microenvironment and exert immunosuppressive effects. On April 18, 2023, we presented preclinical data at the Association for Cancer Research (“AACR”) Annual Meeting that depicted Actimab-A’s role in the tumor microenvironment to overcome immunosuppression driven by MDSCs. We believe that our findings thus far show Actimab-A’s potential to selectively deplete MDSCs in lung and colorectal cancer. Actimab-A also demonstrated superior depletion of human MDSCs compared to Mylotarg, a CD33-targeted antibody-drug conjugate (“ADC”) in colorectal cancer (p<0.01), highlighting the powerful cytotoxicity and potential therapeutic benefit of radiotherapy compared to naked antibodies or ADCs. We believe that the data we have gathered to-date continues to support our objective to demonstrate the potential for Actimab-A to be a backbone therapy to broadly improve antitumor activity of immunotherapies and other therapeutic modalities.
Our differentiated R&D efforts are further exemplified by our next-generation Iomab-ACT conditioning program for rapidly growing cell and gene therapies, as well as our solid tumor and immunotherapy collaborations with Astellas Pharma Inc. (“Astellas”), AVEO Oncology/LG Chem (“LG Chem”) and EpicentRx, Inc. (“EpicentRx”). We have several ongoing programs in solid tumors at the pre-clinical stage with investigational new drug (“IND”) enabling studies underway.
Our platform has been used to develop a pipeline of novel radiotherapeutic assets to drive company growth. Preclinical pharmacology studies with our targeted radiotherapeutics, such as HER3-ARC, HER2-ARC or CD33-ARC, have shown strong improvement in tumor growth inhibition in various preclinical tumor models as single agents or in combination with immunotherapy such as magrolimab, an anti-CD47 monoclonal antibody. These results have prompted the team to spearhead efforts in multiple solid tumor programs.
Actinium’s lead solid tumor program is a targeted radiotherapy against HER3, a pan-cancer target that is overexpressed in several solid tumor indications with high unmet need. We have aligned our R&D strategy of advancing solid tumors into the clinic with our Actimab-A program. We recently presented pre-clinical data at the AACR 2023 Annual Meeting, highlighting this opportunity. We believe the results support further testing to evaluate the anti-tumor effects of HER3-targeted radiotherapy. HER3 conjugated to either alpha-emitting Ac-225 or beta-emitting Lutetium-177 (“Lu-177”) displayed anticancer activity in ovarian and colorectal cancer preclinical models. The consistent overexpression of HER3 in multiple solid tumor types, including ovarian, renal, prostate, urothelial, breast, and lung cancers suggests broad utility of a HER3-targeted agent in a clinical setting, which we intend to explore further.
Our intellectual property (“IP”) portfolio includes over 200 issued patents and pending patent applications worldwide.
Corporate and Other Information
We were organized as a corporation in the State of Nevada in October 1997 and reorganized as a corporation in the State of Delaware in March 2013. Our principal executive offices are located at 100 Park Ave., 23rd Floor, New York, NY 10017. Our telephone number is (646) 677-3870. Our website address is www.actiniumpharma.com. Information accessed through our website is not incorporated into this prospectus and is not a part of this prospectus.
The Securities We May Offer
We may offer up to $500,000,000 of common stock, preferred stock, warrants, units and/or subscription rights in one or more offerings and in any combination. This prospectus provides you with a general description of the securities we may offer. A prospectus supplement, which we will provide each time we offer securities, will describe the specific amounts, prices and terms of these securities.
3
Common Stock
We may issue shares of our common stock from time to time. The holders of our common stock are entitled to one vote per share. Our certificate of incorporation does not provide for cumulative voting. Our directors are divided into three classes. At each annual meeting of stockholders, directors elected to succeed those directors whose terms expire are elected for a term of office to expire at the third succeeding annual meeting of stockholders after their election. The holders of our common stock are entitled to receive ratably such dividends, if any, as may be declared by our board of directors out of legally available funds; however, the current policy of our board of directors is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally available for distribution. The holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of our board of directors and issued in the future.
Our common stock is listed on the NYSE American under the symbol “ATNM.”
Preferred Stock
We may issue shares of our preferred stock from time to time, in one or more series. Our board of directors will determine the rights, preferences, stated values, qualifications or limitations, without any further vote or action by stockholders. Convertible preferred stock will be convertible into our common stock or exchangeable for our other securities. Conversion may be mandatory or at your option or both and would be at prescribed conversion rates.
If we sell any series of preferred stock under this prospectus and applicable prospectus supplements, we will fix the rights, preferences, privileges and restrictions of the preferred stock of such series in the certificate of designation relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the Securities and Exchange Commission, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of the related series of preferred stock. We urge you to read the applicable prospectus supplement related to the series of preferred stock being offered, as well as the complete certificate of designation that contains the terms of the applicable series of preferred stock.
Warrants
We may issue warrants for the purchase of common stock or preferred stock in one or more series. We may issue warrants independently or together with common stock or preferred stock, and the warrants may be attached to or separate from these securities. We will evidence each series of warrants by warrant certificates that we will issue under a separate agreement. We may enter into warrant agreements with a bank or trust company that we select to be our warrant agent. We will indicate the name and address of the warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
In this prospectus, we have summarized certain general features of the warrants. We urge you, however, to read the applicable prospectus supplement related to the particular series of warrants being offered, as well as the warrant agreements and warrant certificates that contain the terms of the warrants. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the Securities and Exchange Commission, the form of warrant agreement or warrant certificate containing the terms of the warrants we are offering before the issuance of the warrants.
Units
We may issue units consisting of common stock, preferred stock and/or warrants for the purchase of common stock or preferred stock in one or more series. In this prospectus, we have summarized certain general features of the units. We urge you, however, to read the applicable prospectus supplement related to the series of units being offered, as well as the unit agreements that contain the terms of the units. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference reports that we file with the Securities and Exchange Commission, the form of unit agreement and any supplemental agreements that describe the terms of the series of units we are offering before the issuance of the related series of units.
Subscription Rights
We may issue subscription rights to purchase shares of common stock or other securities. These subscription rights may be issued independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference reports that we file with the Securities and Exchange Commission, the form of such agreement and any supplemental agreements that describe the terms of the subscription rights we are offering before the issuance of such subscription rights.
4
An investment in our securities involves a high degree of risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the risks applicable to an investment in our securities. Before deciding whether to invest in our securities, you should carefully consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under Item 1A, “Risk Factors,” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which is incorporated herein by reference, as updated or superseded by the risks and uncertainties described under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus and any prospectus supplement related to a particular offering. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, business prospects, financial condition or results of operations could be seriously harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
5
Special Note Regarding Forward-Looking Statements
This prospectus, each prospectus supplement and the information incorporated by reference in this prospectus and each prospectus supplement contain “forward-looking statements,” which include information relating to future events, future financial performance, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and will probably not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or our management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| ● | our history of recurring losses and negative cash flows from operating activities, significant future commitments and the uncertainty regarding the adequacy of our liquidity to pursue our complete business objectives; |
| ● | our ability to complete clinical trials as anticipated and obtain and maintain regulatory approvals for our products; |
| ● | our ability to adequately protect our intellectual property; |
| ● | disputes over ownership of intellectual property; |
| ● | our dependence on a single manufacturing facility and our ability to comply with stringent manufacturing quality standards and to increase production as necessary; |
| ● | the risk that the data collected from our current and planned clinical trials may not be sufficient to demonstrate that our products are an attractive alternative to other procedures and products; |
| ● | intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do; |
| ● | entry of new competitors and products and potential technological obsolescence of our products; |
| ● | loss of a key customer or supplier; |
| ● | adverse economic conditions; |
| ● | adverse federal, state and local government regulation, in the United States; |
| ● | price increases for supplies and components; |
| ● | inability to carry out research, development and commercialization plans; |
| ● | loss or retirement of key executives and research scientists; |
| ● | our ability to maintain compliance with the continued listing requirements of the NYSE American and the risk that our common stock will be delisted if we cannot do so; |
| ● | the geographic, social and economic impact of health epidemics, including the global COVID-19 pandemic, on the Company’s business and liquidity; and |
| ● | other factors discussed in this prospectus. |
You should review carefully the section entitled “Risk Factors” beginning on page 5 of this prospectus for a discussion of these and other risks that relate to our business and investing in our securities. The forward-looking statements contained or incorporated by reference in this prospectus or any prospectus supplement are expressly qualified in their entirety by this cautionary statement. We do not undertake any obligation to publicly update any forward-looking statement to reflect events or circumstances after the date on which any such statement is made or to reflect the occurrence of unanticipated events.
6
Unless we specify another use in the applicable prospectus supplement, we will use the net proceeds from the sale of the securities offered by us for general corporate purposes, which may include, among other things, debt repayment, working capital and/or capital expenditures.
We may also use such proceeds to fund acquisitions of product candidates or technologies that complement our current business. We may set forth additional information on the use of net proceeds from the sale of the securities we offer under this prospectus in a prospectus supplement related to a specific offering.
Investors are cautioned, however, that expenditures may vary substantially from these uses. Investors will be relying on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including the amount of cash generated by our operations, the amount of competition and other operational factors. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes.
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized. Circumstances that may give rise to a change in the use of proceeds include:
| ● | a change in development plan or strategy; |
| ● | delays or difficulties with our clinical trials; |
| ● | negative results from our clinical trials; |
| ● | difficulty obtaining regulatory approval; |
| ● | failure to achieve sales as anticipated; |
| ● | the addition of new product candidates or technologies; |
| ● | our ability to negotiate definitive agreement with acquisition candidates; |
| ● | the availability and terms of debt financing to fund a portion of the purchase price(s) for potential acquisitions; and |
| ● | the availability of other sources of cash including cash flow from operations and new bank debt financing arrangements, if any. |
Pending other uses, we intend to invest the proceeds to us in investment-grade, interest-bearing securities such as money market funds, certificates of deposit, or direct or guaranteed obligations of the U.S. government, or hold as cash. We cannot predict whether the proceeds invested will yield a favorable, or any, return.
7
The following description of common stock and preferred stock summarizes the material terms and provisions of the common stock and preferred stock that we may offer under this prospectus, but is not complete. For the complete terms of our common stock and preferred stock, please refer to our certificate of incorporation, as amended and our amended and restated bylaws, as may be amended from time to time. While the terms we have summarized below will apply generally to any future common stock or preferred stock that we may offer, we will describe the specific terms of any series of preferred stock in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any preferred stock we offer under that prospectus supplement may differ from the terms we describe below.
We have authorized 1,050,000,000 shares of capital stock, par value $0.001 per share, of which 1,000,000,000 are shares of common stock and 50,000,000 are shares of preferred stock. On August 7, 2023, there were 26,997,587 shares of common stock issued and outstanding and no shares of preferred stock issued and outstanding. The authorized and unissued shares of common stock and the authorized and undesignated shares of preferred stock are available for issuance without further action by our stockholders, unless such action is required by applicable law or the rules of any stock exchange on which our securities may be listed.
We also have warrants that are outstanding, which are described below.
Common Stock
The holders of our common stock are entitled to one vote per share. Our Certificate of Incorporation does not provide for cumulative voting. Our directors are divided into three classes. At each annual meeting of stockholders, directors elected to succeed those directors whose terms expire are elected for a term of office to expire at the third succeeding annual meeting of stockholders after their election. The holders of our common stock are entitled to receive ratably such dividends, if any, as may be declared by our board of directors out of legally available funds. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally available for distribution. There are no preemptive, subscription, conversion rights, redemption or sinking fund provisions regarding the common stock. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of our board of directors and issued in the future.
The transfer agent and registrar for our common stock is Securities Transfer Corporation. The transfer agent’s address is 2901 N. Dallas Parkway, Suite 380, Plano, Texas 75093. Our common stock is listed on the NYSE American under the symbol “ATNM.”
Preferred Stock
The board of directors is authorized, subject to any limitations prescribed by law, without further vote or action by the stockholders, to issue from time to time up to 50,000,000 shares of preferred stock in one or more series. Each such series of preferred stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by the board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences, conversion rights and preemptive rights. Issuance of preferred stock by our board of directors may result in such shares having dividend and/or liquidation preferences senior to the rights of the holders of our common stock and could dilute the voting rights of the holders of our common stock.
Prior to the issuance of shares of each series of preferred stock, the board of directors is required by the Delaware General Corporation Law and our Certificate of Incorporation to adopt resolutions and file a certificate of designation with the Secretary of State of the State of Delaware. The certificate of designation fixes for each class or series the designations, powers, preferences, rights, qualifications, limitations and restrictions, including, but not limited to, some or all of the following:
| ● | the number of shares constituting that series and the distinctive designation of that series, which number may be increased or decreased (but not below the number of shares then outstanding) from time to time by action of the board of directors; |
8
| ● | the dividend rate and the manner and frequency of payment of dividends on the shares of that series, whether dividends will be cumulative, and, if so, from which date; |
| ● | whether that series will have voting rights, in addition to any voting rights provided by law, and, if so, the terms of such voting rights; |
| ● | whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the board of directors may determine; |
| ● | whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption; |
| ● | whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund; |
| ● | whether or not the shares of the series will have priority over or be on a parity with or be junior to the shares of any other series or class in any respect; |
| ● | the rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights or priority, if any, of payment of shares of that series; and |
| ● | any other relative rights, preferences and limitations of that series. |
Once designated by our board of directors, each series of preferred stock may have specific financial and other terms.
Anti-Takeover Law and Provisions of Our Certificate of Incorporation and Bylaws
Provisions of our Certificate of Incorporation and Bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our Certificate of Incorporation and Bylaws:
| ● | permits our board of directors to issue up to 50,000,000 shares of preferred stock, without further action by the stockholders, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in control; |
| ● | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors in office; |
| ● | divide our board of directors into three classes, with each class serving staggered three-year terms; |
| ● | do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
| ● | provide that special meetings of our stockholders may be called only by our board of directors, chairman or chief executive officer; and |
| ● | provide advance notice provisions with which a stockholder who wishes to nominate a director or propose other business to be considered at a stockholder meeting must comply. |
9
As of August 7, 2023, there were 1,444,733 shares of common stock that may be issued upon the exercise of outstanding warrants.
We may issue warrants for the purchase of common stock or preferred stock in one or more series. We may issue warrants independently or together with common stock or preferred stock, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants by warrant certificates that we may issue under a separate agreement. We may enter into a warrant agreement with a warrant agent. Each warrant agent may be a bank or transfer agent that we select which has its principal office in the United States. We may also choose to act as our own warrant agent. We will indicate the name and address of any such warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the terms of the series of warrants, including:
| ● | the offering price and aggregate number of warrants offered; |
| ● | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| ● | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| ● | in the case of warrants to purchase common stock or preferred stock, the number or amount of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which and currency in which these shares may be purchased upon such exercise; |
| ● | the manner of exercise of the warrants, including any cashless exercise rights; |
| ● | the warrant agreement under which the warrants will be issued; |
| ● | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; |
| ● | anti-dilution provisions of the warrants, if any; |
| ● | the terms of any rights to redeem or call the warrants; |
| ● | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| ● | the dates on which the right to exercise the warrants will commence and expire or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will be exercisable; |
| ● | the manner in which the warrant agreement and warrants may be modified; |
| ● | the identities of the warrant agent and any calculation or other agent for the warrants; |
| ● | federal income tax consequences of holding or exercising the warrants; |
| ● | the terms of the securities issuable upon exercise of the warrants; |
| ● | any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed or quoted; and |
| ● | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including, in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
10
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to 5:00 P.M. eastern time, the close of business, on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required exercise price by the methods provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate, and in the applicable prospectus supplement, the information that the holder of the warrant will be required to deliver to the warrant agent.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants.
Enforceability of Rights By Holders of Warrants
Any warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action the holder’s right to exercise, and receive the securities purchasable upon exercise of, its warrants in accordance with their terms.
Warrant Agreement Will Not Be Qualified Under Trust Indenture Act
No warrant agreement will be qualified as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Governing Law
Each warrant agreement and any warrants issued under the warrant agreements will be governed by New York law.
11
We may issue units comprised of one or more of the other securities described in this prospectus or any prospectus supplement in any combination. Each unit will be issued so that the holder of the unit is also the holder, with the rights and obligations of a holder, of each security included in the unit. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any times before a specified date or upon the occurrence of a specified event or occurrence.
The applicable prospectus supplement will describe:
| ● | the designation and the terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| ● | any unit agreement under which the units will be issued; |
| ● | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| ● | whether the units will be issued in fully registered or global form. |
12
Description of Subscription Rights
The following is a general description of the terms of the subscription rights we may issue from time to time. Particular terms of any subscription rights we offer will be described in the prospectus supplement relating to such subscription rights, and may differ from the terms described herein.
We may issue subscription rights to purchase shares of common stock or other securities offered hereby. These subscription rights may be issued independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The applicable prospectus supplement will describe the specific terms of any offering of subscription rights for which this prospectus is being delivered, including the following:
| ● | whether common stock or other securities will be offered under the stockholder subscription rights; |
| ● | the price, if any, for the subscription rights; |
| ● | the exercise price payable for each security upon the exercise of the subscription rights; |
| ● | the number of subscription rights issued to each stockholder; |
| ● | the number and terms of the securities which may be purchased per each subscription right; |
| ● | the extent to which the subscription rights are transferable; |
| ● | any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights; |
| ● | the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire; |
| ● | the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities; |
| ● | if appropriate, a discussion of material U.S. federal income tax considerations; and |
| ● | if applicable, the material terms of any standby underwriting or purchase arrangement entered into by us in connection with the offering of subscription rights. |
The description in the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by reference to the applicable subscription rights certificate or subscription rights agreement, which will be filed with the Securities and Exchange Commission if we offer subscription rights.
13
We may sell the securities being offered pursuant to this prospectus to or through underwriters, through dealers, through agents, or directly to one or more purchasers or through a combination of these methods. The applicable prospectus supplement will describe the terms of the offering of the securities, including:
| ● | the name or names of any underwriters, if any, and if required, any dealers or agents; |
| ● | the purchase price of the securities and the proceeds we will receive from the sale; |
| ● | any underwriting discounts and other items constituting underwriters’ compensation; |
| ● | any discounts or concessions allowed or reallowed or paid to dealers; and |
| ● | any securities exchange or market on which the securities may be listed or traded. |
We may distribute the securities from time to time in one or more transactions at:
| ● | a fixed price or prices, which may be changed; |
| ● | market prices prevailing at the time of sale, directly by us or through a designated agent; |
| ● | in “at the market” offerings within the meaning of Rule 415 under the Securities Act of 1933, as amended, or through a market maker or into an existing market, on an exchange, or otherwise; |
| ● | prices related to such prevailing market prices; |
| ● | in block trades in which a broker-dealer will attempt to sell the securities as agent, but may position and resell a portion of the block as principal to facilitate the transaction; or |
| ● | negotiated prices. |
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement.
We may also make direct sales through subscription rights distributed to our existing shareholders on a pro rata basis, which may or may not be transferable. In any distribution of subscription rights to our shareholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the unsubscribed securities to third parties. In addition, whether or not all of the underlying securities are subscribed for, we may concurrently offer additional securities to third parties directly or through underwriters, dealers or agents.
If underwriters are used in an offering, we will execute an underwriting agreement with such underwriters and will specify the name of each underwriter and the terms of the transaction (including any underwriting discounts and other terms constituting compensation of the underwriters and any dealers) in a prospectus supplement. The securities may be offered to the public either through underwriting syndicates represented by managing underwriters or directly by one or more investment banking firms or others, as designated. If an underwriting syndicate is used, the managing underwriter(s) will be specified on the cover of the prospectus supplement. If underwriters are used in the sale, the offered securities will be acquired by the underwriters for their own accounts and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may be changed from time to time. Unless otherwise set forth in the prospectus supplement, the obligations of the underwriters to purchase the offered securities will be subject to conditions precedent, and the underwriters will be obligated to purchase all of the offered securities, if any are purchased.
We may grant to the underwriters options to purchase additional securities to cover over-allotments, if any, at the public offering price, with additional underwriting commissions or discounts, as may be set forth in a related prospectus supplement. The terms of any over-allotment option will be set forth in the prospectus supplement for those securities.
14
If we use a dealer in the sale of the securities being offered pursuant to this prospectus or any prospectus supplement, we will sell the securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. The names of the dealers and the terms of the transaction will be specified in a prospectus supplement.
We may sell the securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, any agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
In connection with the sale of the securities, underwriters, dealers or agents may receive compensation from us or from purchasers of the securities for whom they act as agents, in the form of discounts, concessions or commissions. Underwriters may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of the securities, and any institutional investors or others that purchase securities directly for the purpose of resale or distribution, may be deemed to be underwriters, and any discounts or commissions received by them from us and any profit on the resale of the common stock by them may be deemed to be underwriting discounts and commissions under the Securities Act of 1933, as amended.
We may provide agents, underwriters and other purchasers with indemnification against particular civil liabilities, including liabilities under the Securities Act of 1933, as amended, or contribution with respect to payments that the agents, underwriters or other purchasers may make with respect to such liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
To facilitate the public offering of a series of securities, persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise affect the market price of the securities. This may include over-allotments or short sales of the securities, which involves the sale by persons participating in the offering of more securities than have been sold to them by us. In addition, those persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to underwriters or dealers participating in any such offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as to the direction or magnitude of any effect that the transactions described above, if implemented, may have on the price of our securities.
Unless otherwise specified in the applicable prospectus supplement, any common stock sold pursuant to a prospectus supplement will be eligible for listing on the NYSE American, subject to official notice of issuance. Any underwriters to whom securities are sold by us for public offering and sale may make a market in the securities, but such underwriters will not be obligated to do so and may discontinue any market making at any time without notice.
In order to comply with the securities laws of some states, if applicable, the securities offered pursuant to this prospectus will be sold in those states only through registered or licensed brokers or dealers. In addition, in some states securities may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and complied with.
15
The validity of the securities offered by this prospectus will be passed upon by Haynes and Boone, LLP, New York, New York.
The consolidated financial statements of Actinium Pharmaceuticals, Inc. as of and for the years ended December 31, 2022 and 2021 appearing in Actinium Pharmaceuticals, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Marcum LLP, as set forth in its report thereon, including therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
Where You Can Find More Information
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, and in accordance therewith file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission. The Securities and Exchange Commission maintains a website that contains such reports, proxy and information statements and other information regarding registrants that file electronically with the Securities and Exchange Commission. The address of the Securities and Exchange Commission’s website is www.sec.gov.
We make available free of charge on or through our website at www.actiniumpharma.com, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with or otherwise furnish it to the Securities and Exchange Commission.
We have filed with the Securities and Exchange Commission a registration statement under the Securities Act of 1933, as amended, relating to the offering of these securities. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement, at prescribed rates, from the Securities and Exchange Commission at the address listed above, or for free at www.sec.gov. The registration statement and the documents referred to below under “Incorporation of Certain Information By Reference” are also available on our website, www.actiniumpharma.com.
We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
16
Incorporation of Certain Information by Reference
The Securities and Exchange Commission allows us to “incorporate by reference” the information we have filed with it, which means that we can disclose important information to you by referring you to those documents. The information we incorporate by reference is an important part of this prospectus, and later information that we file with the Securities and Exchange Commission will automatically update and supersede this information. We incorporate by reference the documents listed below and any future documents (excluding information furnished pursuant to Items 2.02 and 7.01 of Form 8-K) we file with the Securities and Exchange Commission pursuant to Sections l3(a), l3(c), 14 or l5(d) of the Securities Exchange Act of 1934, as amended, subsequent to the date of this prospectus and prior to the termination of the offering:
| ● | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the Securities and Exchange Commission on March 31, 2023; |
| ● | Our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2023, filed with the Securities and Exchange Commission on May 15, 2023; and |
| ● | The description of the Company’s common stock and warrants contained in Exhibit 4.15 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the Securities and Exchange Commission on March 31, 2021, including any amendments thereto or reports filed for the purposes of updating this description. |
All filings filed by us pursuant to the Securities Exchange Act of 1934, as amended, after the date of the initial filing of this registration statement and prior to the effectiveness of such registration statement (excluding information furnished pursuant to Items 2.02 and 7.01 of Form 8-K) shall also be deemed to be incorporated by reference into the prospectus.
You should rely only on the information incorporated by reference or provided in this prospectus. We have not authorized anyone else to provide you with different information. Any statement contained in a document incorporated by reference into this prospectus will be deemed to be modified or superseded for the purposes of this prospectus to the extent that a later statement contained in this prospectus or in any other document incorporated by reference into this prospectus modifies or supersedes the earlier statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date of this prospectus or the date of the documents incorporated by reference in this prospectus.
We will provide without charge to each person to whom a copy of this prospectus is delivered, upon written or oral request, a copy of any or all of the reports or documents that have been incorporated by reference in this prospectus but not delivered with this prospectus (other than an exhibit to these filings, unless we have specifically incorporated that exhibit by reference in this prospectus). Any such request should be addressed to us at:
Actinium Pharmaceuticals, Inc.
Attn: Steve O’Loughlin
100 Park Ave., 23rd Floor
New York, NY 10017
(646) 677-3870
You may also access the documents incorporated by reference in this prospectus through our website at www.actiniumpharma.com. Except for the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated in this prospectus or the registration statement of which it forms a part.
17
$500,000,000

Common Stock
Preferred Stock|
Warrants
Units
Subscription Rights
PROSPECTUS
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 11, 2023
Prospectus

Actinium Pharmaceuticals, Inc.
Up to $200,000,000
Common Stock
We previously entered into an Amended and Restated Capital on Demand™ Sales Agreement, or the sales agreement, with JonesTrading Institutional Services LLC, or JonesTrading, and B. Riley Securities, Inc., or B. Riley Securities, dated June 28, 2022, relating to the sale of shares of our common stock, par value $0.001 per share, from time to time through or to JonesTrading or B. Riley Securities, acting as agents or principals. In accordance with the terms of the sales agreement, pursuant to this prospectus and the accompanying base prospectus, we may offer and sell our common stock having an aggregate offering price of up to $200,000,000 from time to time through or to JonesTrading and B.Riley Securities. To date, we have sold an aggregate of 11,463,972 shares pursuant to the sales agreement under a registration statement on Form S-3 (File No. 333-242322) filed on August 7, 2020, and declared effective on August 14, 2020, for aggregate gross proceeds of approximately $93.9 million.
Sales of our common stock, if any, under this prospectus will be made by any method permitted that is deemed an “at the market offering” as defined in Rule 415 under the Securities Act of 1933, as amended, or the Securities Act. JonesTrading and B. Riley Securities, referred to herein as the agents, will act as our sales agents using commercially reasonable efforts consistent with their normal trading and sales practices. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
The agents will be entitled to compensation at a commission rate up to 3.0% of the gross sales price per share sold under the sales agreement. See “Plan of Distribution” beginning on page 11 for additional information regarding the compensation to be paid to the agents. In connection with the sale of the shares of common stock on our behalf, each agent will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of each agent will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to the agent with respect to certain liabilities, including liabilities under the Securities Act.
Investing in our securities involves a high degree of risk. These risks are discussed in this prospectus under “Risk Factors” beginning on page 5 and in the documents incorporated by reference into this prospectus.
Our common stock is listed on the NYSE American under the symbol “ATNM.” On August 10, 2023, the last reported sale price of our common stock was $6.58 per share.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
JonesTrading B. Riley Securities
The date of this prospectus is , 2023.
Table of Contents
i
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission using a “shelf” registration process. This prospectus relates to the offering of our common stock. Before buying any of the common stock that we are offering, we urge you to carefully read this prospectus, together with the information incorporated by reference as described under the heading “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.” These documents contain important information that you should consider when making your investment decision.
This prospectus describes the specific terms of the common stock we are offering and also adds to and updates information contained in the documents incorporated by reference into this prospectus. To the extent there is a conflict between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated by reference in this prospectus, on the other hand, you should rely on the information in this prospectus. If any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference into this prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
You should only rely on the information contained or incorporated by reference in this prospectus and any issuer free writing prospectus that we may authorize for use in connection with this offering. No person has been authorized to give any information or make any representations in connection with this offering other than those contained or incorporated by reference in this prospectus and any related issuer free writing prospectus in connection with the offering described herein and therein, and, if given or made, such information or representations must not be relied upon as having been authorized by us. Neither this prospectus nor any related issuer free writing prospectus shall constitute an offer to sell or a solicitation of an offer to buy offered securities in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. This prospectus does not contain all of the information included in the registration statement. For a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits.
You should read the entire prospectus and any related issuer free writing prospectus, as well as the documents incorporated by reference into this prospectus or any related issuer free writing prospectus, before making an investment decision. Neither the delivery of this prospectus or any issuer free writing prospectus nor any sale made hereunder shall under any circumstances imply that the information contained or incorporated by reference herein or in any issuer free writing prospectus is correct as of any date subsequent to the date hereof or of such issuer free writing prospectus. You should assume that the information appearing in this prospectus or any document incorporated by reference is accurate only as of the date of the applicable documents, regardless of the time of delivery of this prospectus or any sale of securities. Our business, financial condition, results of operations and prospects may have changed since that date.
ii
The items in the following summary are described in more detail later in this prospectus and in the accompanying prospectus. This summary provides an overview of selected information and does not contain all the information you should consider before investing in our common stock. Therefore, you should read the entire prospectus and the accompanying prospectus carefully, including the “Risk Factors” section, and other documents or information included or incorporated by reference in this prospectus and the accompanying prospectus before making any investment decision. As used in this prospectus, unless the context otherwise indicates, the terms “we,” “our,” “us,” or “the Company” refer to Actinium Pharmaceuticals, Inc., a Delaware corporation, and its subsidiaries taken as a whole.
The Company
Actinium Pharmaceuticals, Inc. (“Actinium”) develops targeted radiotherapies intended to meaningfully improve survival for patients with relapsed or refractory cancer who have failed existing therapies. Our vision is to build a specialty, hospital-focused, radiotherapeutics company that develops and markets medicines for patients who are treated primarily in large quaternary care hospitals and their catchment areas.
We intend to leverage the clinical data of our lead product candidates, Iomab-B and Actimab-A, to improve outcomes in patients with relapsed or refractory acute myeloid leukemia (“r/r AML”) by launching two radiotherapy drugs over the next several years to address the significant need for better outcomes from treatment with therapeutics or from undergoing a bone marrow transplant (“BMT”).
We also intend to further advance Iomab-B outside of acute myeloid leukemia (“AML”) based on promising data as a disease control and conditioning agent for various other blood cancers. Based on early promising clinical trial results, we are also working on a lower dose, next generation conditioning program, Iomab-ACT, for rapidly growing cell and gene therapies.
Our Clinical Pipeline
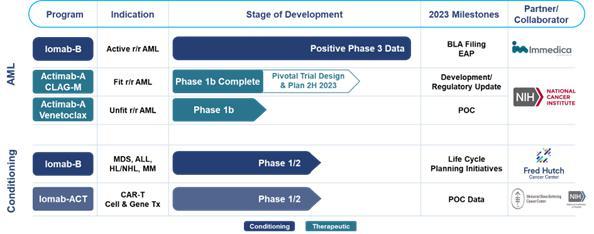
AML is an aggressive, heterogeneous disease that is difficult-to-treat. Most AML patients develop relapsed or refractory disease within one year of being afflicted and have an extremely poor prognosis and dismal survival. Currently, a BMT is the only curative regimen available for AML patients, however, access is limited to AML patients who are fit enough to withstand the challenges associated with this treatment. The majority of AML patients are considered not transplantable in routine clinical practice as they are not fit enough to withstand the rigors of the patient journey which includes therapy to attain a remission, conditioning regimens to destroy diseased marrow, challenge of the transplant itself or post-transplant complications.
1
Our Iomab-B and Actimab-A product candidates potentially fill the major unmet medical needs in r/r AML in a complementary fashion as they are directed at different parts of the patient journey. Iomab-B is being developed as a targeted bridging therapy candidate that we believe could provide both disease control and conditioning in one agent. We believe results from our Phase 3 SIERRA trial demonstrate the possibility for unprecedented access to a BMT and improved survival in unfit patients who are currently not considered transplantable in routine clinical practice. We are developing Actimab-A as a targeted therapy candidate for fit patients. Actimab-A has demonstrated an extension in survival in a proof-of-concept study and is poised for advanced development in collaboration with the NCI, or National Cancer Institute (“NCI”). Together, we believe these two product candidates could provide us the opportunity to transform the treatment of AML, especially in the relapsed and refractory segment which represents over 50% of AML patients.
We announced in October 2022 that Iomab-B met the primary endpoint of durable Complete Remission (“dCR”) with a high degree of statistical significance (p<0.0001) in the pivotal Phase 3 Study of Iomab-B in Elderly Relapsed or Refractor AML, or “SIERRA trial.” In February 2023, we announced full trial results, demonstrating unprecedented transplant access and improved outcomes in patients with r/r AML, with double 1-year and median overall survival (“OS”) compared to control arm patients. These data were presented at the 2023 Tandem Meetings aka the Transplantation & Cellular Therapy (“TCT”) Meetings of the American Society for Transplantation and Cellular Therapy (“ASTCT”) and the Center for International Blood & Marrow Transplant Research (“CIBMTR”). We believe these results from the SIERRA trial may provide the opportunity, if we are able to obtain U.S. Food and Drug Administration (“FDA”) approval, to establish Iomab-B as a new standard of care.
The results from the SIERRA trial were recently presented and discussed at major bone marrow transplant and hematology medical conferences, nuclear medicines conference and nursing congress, which is helping to broaden the awareness of Iomab-B among members of the relevant medical and scientific communities as we prepare for potential commercialization if we achieve FDA approval. Including TCT, the SIERRA Phase 3 results have now been highlighted in oral presentations at four international medical conferences in the U.S. and EU attended by key Iomab-B stakeholders including bone marrow transplant physicians, hematologists and nuclear medicine physicians. On April 27, 2023, the results from the SIERRA trial were also showcased at the European Society for Blood and Marrow Transplantation (“EBMT”) 49th Annual Meeting, which was well-attended by the European transplant community. Also in April 2023, two posters that detailed clinical findings from the SIERRA trial sites were presented at the 48th Annual Oncology Nursing Society (“ONS”) Congress. We believe that the scientific community present at such events took note of the successful administration of Iomab-B infusions at various BMT centers, which was done without increasing radiation exposure risks to treating nursing staff. SIERRA results were also awarded an oral presentation at the European Hematology Association (“EHA”) 2023 Hybrid Congress and presented in Frankfurt, Germany on June 10, 2023. The SIERRA results were also presented at the Society for Nuclear Medicine and Molecule Imaging (“SNMMI”) annual meeting on June 26, 2023 and was awarded the Henry N. Wagner, Jr., Abstract of the Year award, which represents the top selection out of more than 1,500 abstracts accepted for presentation.
We are actively working on launching an early access program (“EAP”) for Iomab-B. We are also working towards completing and submitting our Biologics License Application (“BLA”) for Iomab-B to the FDA by the end of 2023 and if approved, we intend to commercialize Iomab-B in the U.S. We are committed to bringing Iomab-B to patients globally and are working with Immedica AB (“Immedica”), our European, Middle East and North Africa (“EUMENA”) partner, for the subsequent marketing authorization application (“MAA”) of Iomab-B with the European Medicines Agency (“EMA”). Europe represents a large commercial market opportunity with approximately twice as many transplants performed in Europe compared to the U.S.
2
Actimab-A is being developed under what we believe to be the current industry-leading clinical-study program utilizing the potent alpha radiation emitting isotope Actinium-225 (“Ac-225”) with clinical data in approximately 150 patients treated over six clinical trials. The potent linear energy transfer emitted by Ac-225 has no known resistance mechanism. Actimab-A is being developed in combination with other regimens to exploit mechanistic synergies and leverage the mutation-agnostic mechanism of action of Ac-225 with the objective of establishing it as a backbone therapy in AML, an extremely heterogenous disease.
We believe our Actimab-A + CLAG-M therapeutic combination trial results in r/r AML provide validation of this approach. Such results were presented at the American Society of Hematology (“ASH”) 2022 Annual Meeting & Exposition in December 2022. Phase 1 results from the Actimab-A + CLAG-M combination trial showed high response rates and minimal residual disease (“MRD”) negativity, translating to a survival benefit of 53% and 32% at one and two years in patients who are typically expected to live two to four months. At the same meeting, we shared Phase 1 data showing that the combination of Actimab-A + venetoclax was well-tolerated with responses, including a Complete Remission (“CR”) and a partial response in early dose escalation cohorts. We believe the promise of these results have paved the way for the NCI Cooperative Research and Development Agreement (“CRADA”), announced on February 6, 2023, to develop Actimab-A for the treatment of patients with AML and other hematologic malignancies. Under the CRADA, Actinium expects to initiate the late-stage development of Actimab-A, including a potential pivotal trial, in combination as a backbone therapy for r/r AML and expects to update on the program and timing by end of the second half of 2023.
To explore the potential for a broader development opportunity with our Actimab-A program, we are studying the potential use of Actimab-A in solid tumor indications through our R&D efforts. CD33-expressing myeloid derived suppressor cells (“MDSCs”) are present within the tumor microenvironment and exert immunosuppressive effects. On April 18, 2023, we presented preclinical data at the Association for Cancer Research (“AACR”) Annual Meeting that depicted Actimab-A’s role in the tumor microenvironment to overcome immunosuppression driven by MDSCs. We believe that our findings thus far show Actimab-A’s potential to selectively deplete MDSCs in lung and colorectal cancer. Actimab-A also demonstrated superior depletion of human MDSCs compared to Mylotarg, a CD33-targeted antibody-drug conjugate (“ADC”) in colorectal cancer (p<0.01), highlighting the powerful cytotoxicity and potential therapeutic benefit of radiotherapy compared to naked antibodies or ADCs. We believe that the data we have gathered to-date continues to support our objective to demonstrate the potential for Actimab-A to be a backbone therapy to broadly improve antitumor activity of immunotherapies and other therapeutic modalities.
Our differentiated R&D efforts are further exemplified by our next-generation Iomab-ACT conditioning program for rapidly growing cell and gene therapies, as well as our solid tumor and immunotherapy collaborations with Astellas Pharma Inc. (“Astellas”), AVEO Oncology/LG Chem (“LG Chem”) and EpicentRx, Inc. (“EpicentRx”). We have several ongoing programs in solid tumors at the pre-clinical stage with investigational new drug (“IND”) enabling studies underway.
Our platform has been used to develop a pipeline of novel radiotherapeutic assets to drive company growth. Preclinical pharmacology studies with our targeted radiotherapeutics, such as HER3-ARC, HER2-ARC or CD33-ARC, have shown strong improvement in tumor growth inhibition in various preclinical tumor models as single agents or in combination with immunotherapy such as magrolimab, an anti-CD47 monoclonal antibody. These results have prompted the team to spearhead efforts in multiple solid tumor programs.
Actinium’s lead solid tumor program is a targeted radiotherapy against HER3, a pan-cancer target that is overexpressed in several solid tumor indications with high unmet need. We have aligned our R&D strategy of advancing solid tumors into the clinic with our Actimab-A program. We recently presented pre-clinical data at the AACR 2023 Annual Meeting, highlighting this opportunity. We believe the results support further testing to evaluate the anti-tumor effects of HER3-targeted radiotherapy. HER3 conjugated to either alpha-emitting Ac-225 or beta-emitting Lutetium-177 (“Lu-177”) displayed anticancer activity in ovarian and colorectal cancer preclinical models. The consistent overexpression of HER3 in multiple solid tumor types, including ovarian, renal, prostate, urothelial, breast, and lung cancers suggests broad utility of a HER3-targeted agent in a clinical setting, which we intend to explore further.
Our intellectual property (“IP”) portfolio includes over 200 issued patents and pending patent applications worldwide.
Corporate and Other Information
We were organized as a corporation in the State of Nevada in October 1997 and reorganized as a corporation in the State of Delaware in March 2013. Our principal executive offices are located at 100 Park Ave., 23rd Floor, New York, NY 10017. Our telephone number is (646) 677-3870. Our website address is www.actiniumpharma.com. Information accessed through our website is not incorporated into this prospectus and is not a part of this prospectus.
3
|
Common stock offered by us |
Shares of our common stock having an aggregate offering price of up to $200,000,000. |
| Manner of offering | “At the market offering” as defined in Rule 415(a)(4) under the Securities Act, that may be made from time to time, through or to the agents, each as agent or principal. See section titled “Plan of Distribution” on page 11 of this prospectus. |
| Use of proceeds | We intend to use the net proceeds from this offering for general corporate purposes, including the advancement of our drug candidates in clinical trials, regulatory submissions, potential commercial activity, preclinical research and development, capital expenditures, and to meet working capital needs. Please see “Use of Proceeds” on page 8. |
| Risk factors | Investing in our securities involves a high degree of risk. You should read the section titled “Risk Factors” beginning on page 5 of this prospectus and in the documents incorporated by reference into this prospectus, for a discussion of factors to consider before deciding to invest in our common stock. |
| NYSE American symbol | ATNM. |
4
An investment in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should carefully consider the risks and uncertainties described below, together with the information under the heading “Risk Factors” in our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and in our Quarterly Reports on Form 10-Q, all of which are incorporated herein by reference, as updated or superseded by the risks and uncertainties described under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus together with all of the other information contained or incorporated by reference in this prospectus. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, business prospects, financial condition or results of operations could be seriously harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
Additional Risks Related to this Offering
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a significant return.
Our management will have broad discretion over the use of proceeds from this offering. We currently intend to use the net proceeds of this offering for general corporate purposes, including the advancement of our drug candidates in clinical trials, regulatory submissions, potential commercial activity, preclinical research and development, capital expenditures, and to meet working capital needs. For more information, see “Use of Proceeds” on page 8. However, our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. You will not have the opportunity, as part of your investment decision, to assess whether these proceeds are being used appropriately.
The amount and timing of our actual expenditures will depend upon numerous factors, including the amount of cash generated by our operations, the amount of competition and other operational factors. The costs and timing of development activities, particularly conducting clinical trials and preclinical studies, are highly uncertain, subject to substantial risks and can often change. Depending on the outcome of these activities and other unforeseen events, our plans and priorities may change, and we may apply the net proceeds of this offering in different proportions than we currently anticipate.
Our failure to apply these funds effectively could have a material adverse effect on our business, delay the further development of our product candidates and cause the price of our common shares to decline.
The failure by management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our product candidates.
Resales of our common stock in the public market during this offering by our stockholders may cause the market price of our common stock to fall.
We may issue shares of common stock from time to time in connection with this offering. The issuance from time to time of these new shares of common stock, or our ability to issue new shares of common stock in this offering, could result in resales of our shares of common stock by our current stockholders concerned about the potential dilution of their holdings. In turn, these resales could have the effect of depressing the market price for our common stock.
5
Purchasers in this offering will likely experience immediate and substantial dilution in the book value of their investment.
The shares of common stock sold in this offering, if any, will be sold from time to time at various prices. However, the expected offering price per share of common stock may be substantially higher than the net tangible book value per share of common stock. Therefore, if you purchase shares of our common stock in this offering, your interest will be diluted to the extent of the difference between the price per share you pay and the net tangible book value per share of common stock. Assuming the sale of an aggregate amount of $200,000,000 of shares of our common stock in this offering at an assumed offering price of $6.68 per share, which was the last reported sale price of our common stock on the NYSE American on August 7, 2023, based on our net tangible book value as of March 31, 2023, and a pro forma net tangible book value per share of $2.39 after giving effect to sales of our common stock between April 1, 2023, and August 7, 2023, if you purchase shares of common stock in this offering you will suffer substantial and immediate dilution of approximately $3.21 per share in the net tangible book value of the share common stock. The future exercise or conversion, as applicable, of outstanding options, restricted stock units, warrants and other instruments that are convertible or exercisable into common stock, if any, will result in further dilution of your investment. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase shares of our common stock in this offering.
You may experience future dilution as a result of future equity offerings.
To raise additional capital, we may in the future offer additional shares of common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We may sell common stock or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering.
Sales of a substantial number of shares of our common stock, or the perception that such sales may occur, may adversely impact the price of our common stock.
Almost all of our 26,997,587 outstanding shares of common stock as of August 7, 2023, as well as a substantial number of shares of our common stock underlying outstanding options and warrants, are available for sale in the public market, either pursuant to Rule 144 under the Securities Act, or an effective registration statement. Pursuant to the shelf registration statement on Form S-3 of which this prospectus is a part, we have registered for sale up to $500,000,000 of our equity securities over the next several years. Sales of a substantial number of shares of our common stock in the public markets could depress the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities. We cannot predict the effect that future sales of our common stock would have on the market price of our common stock.
The common stock offered hereby will be sold in “at the market” offerings, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales made at prices lower than the prices they paid.
The actual number of shares we will issue under the sales agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver placement notices to either of the agents at any time throughout the term of the sales agreement. The number of shares that are sold by an agent after delivering a placement notice will fluctuate based on the market price of the common stock during the sales period and limits we set with said agent. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued.
6
Special Note Regarding Forward-Looking Statements
This prospectus and the information incorporated by reference in this prospectus contain “forward-looking statements,” which include information relating to future events, future financial performance, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and will probably not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or our management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| ● | our history of recurring losses and negative cash flows from operating activities, significant future commitments and the uncertainty regarding the adequacy of our liquidity to pursue our complete business objectives; |
| ● | our ability to complete clinical trials as anticipated and obtain and maintain regulatory approvals for our products; |
| ● | our ability to adequately protect our intellectual property; |
| ● | disputes over ownership of intellectual property; |
| ● | our dependence on a single manufacturing facility and our ability to comply with stringent manufacturing quality standards and to increase production as necessary; |
| ● | the risk that the data collected from our current and planned clinical trials may not be sufficient to demonstrate that our products are an attractive alternative to other procedures and products; |
| ● | intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do; |
| ● | entry of new competitors and products and potential technological obsolescence of our products; |
| ● | loss of a key customer or supplier; |
| ● | adverse economic conditions; |
| ● | adverse federal, state and local government regulation, in the United States; |
| ● | price increases for supplies and components; |
| ● | inability to carry out research, development and commercialization plans; |
| ● | loss or retirement of key executives and research scientists; |
| ● | our ability to maintain compliance with the continued listing requirements of the NYSE American and the risk that our common stock will be delisted if we cannot do so; |
| ● | the geographic, social and economic impact of health epidemics, including the global COVID-19 pandemic, on the Company’s business and liquidity; and |
| ● | other factors discussed in this prospectus. |
You should review carefully the section entitled “Risk Factors” beginning on page 5 of this prospectus for a discussion of these and other risks that relate to our business and investing in our securities. The forward-looking statements contained or incorporated by reference in this prospectus are expressly qualified in their entirety by this cautionary statement. Except as required by applicable law, we do not undertake any obligation to publicly update any forward-looking statement contained in this prospectus, the accompanying prospectus or the documents incorporated by reference herein to reflect events or circumstances after the date on which any such statement is made or to reflect the occurrence of unanticipated events. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
7
We may issue and sell shares of common stock having aggregate sales proceeds of up to $200,000,000 from time to time, before deducting sales agent commissions and expenses. The amount of proceeds from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold. There can be no assurance that we will be able to sell any shares under or fully utilize the sales agreement with the agents
We currently intend to use the net proceeds from the sale of securities offered by this prospectus for general corporate purposes, including the advancement of our drug candidates in clinical trials, regulatory submissions, potential commercial activity, preclinical research and development, capital expenditures, and to meet working capital needs.
Investors are cautioned, however, that expenditures may vary substantially from these uses. Investors will be relying on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including the amount of cash generated by our operations, the amount of competition and other operational factors. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes.
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized. Circumstances that may give rise to a change in the use of proceeds include:
| ● | a change in development plan or strategy; |
| ● | the addition of new products or applications; |
| ● | technical delays; |
| ● | delays or difficulties with our clinical trials; |
| ● | negative results from our clinical trials; |
| ● | difficulty obtaining U.S. Food and Drug Administration approval; and |
| ● | the availability of other sources of cash including additional offerings, if any. |
Pending other uses, we intend to invest the proceeds to us in investment-grade, interest-bearing securities such as money market funds, certificates of deposit, or direct or guaranteed obligations of the U.S. government, or hold as cash. We cannot predict whether the proceeds invested will yield a favorable, or any, return.
8
If you invest in our common stock, your interest will be diluted to the extent of the difference between the price per share you pay in this offering and the net tangible book value per share of common stock immediately after this offering. The net tangible book value of our common stock as of March 31, 2023, was approximately $54.7 million, or approximately $2.12 per share of common stock based on 25,729,370 shares of common stock outstanding at that time. “Net tangible book value” is total assets minus the sum of liabilities and intangible assets. “Net tangible book value per share” is net tangible book value divided by the total number of shares outstanding.
After giving effect to the sale of 1,210,965 shares of our common stock pursuant to the sales agreement, and the receipt by the Company net proceeds of approximately $9.8 million, after sales agent fees and expenses payable by us, between April 1, 2023, and August 7, 2023, our pro forma net tangible book value as of March 31, 2023 would have been approximately $64.4 million, or approximately $2.39 per share of our common stock.
After giving further effect to the sale of our common stock in the aggregate amount of $200,000,000 in this offering at an assumed offering price of $6.68 per share, the last reported sale price of our common stock on the NYSE American on August 7, 2023, and after deducting the commissions and estimated offering expenses payable by us, our pro forma, as adjusted net tangible book value as of March 31, 2023, would have been approximately $195.8 million, or approximately $3.41 per share of our common stock. This represents an immediate increase in net tangible book value of $1.02 per share to our existing stockholders and an immediate dilution of approximately $3.27 per share to new investors participating in this offering, as illustrated by the following table:
| Assumed offering price per share of common stock | $ | 6.68 | ||||||
| Historical net tangible book value per share of common stock as of March 31, 2023 | $ | 2.12 | ||||||
| Increase in net tangible book value per share of common stock attributable to issuances of common stock between April 1, 2023, and August 7, 2023 | $ | 0.27 | ||||||
| Pro forma net tangible book value per share of common stock as of March 31, 2023 | $ | 2.39 | ||||||
| Increase in pro forma net tangible book value per share of common stock attributable to the offering | $ | 1.02 | ||||||
| Pro Forma as adjusted net tangible book value per share of common stock as of March 31, 2023 after giving effect to this offering | $ | 3.41 | ||||||
| Dilution in net tangible book value per share of common stock to new investors in the offering | $ | 3.27 |
The pro forma as adjusted information is illustrative only and will adjust based on the actual price to the public, the actual number of shares sold and other terms of the offering determined at the time common stock is sold pursuant to this prospectus. The pro forma as adjusted information assumes that all of our common stock in the aggregate amount of $200,000,000 is sold at the assumed offering price of $6.68 per share, the last reported sale price of our common stock on the NYSE American on August 7, 2023. The shares sold in this offering, if any, will be sold from time to time at various prices.
The discussion and table above are based on 25,729,370 shares of common stock outstanding as of March 31, 2023, and excludes the following potentially dilutive securities as of that date:
| ● | 3,324,311 shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2023, under our equity incentive plans, with a weighted average exercise price of $8.07 per share; |
| ● | 6,455,623 shares of common stock available for future grants under our equity incentive plans as of March 31, 2023; |
| ● | 325,000 shares of common stock issuable upon exercise of vested restricted stock units outstanding as of March 31, 2023, under our equity incentive plans, with a weighted average date of grant price of $5.96 per share, and |
| ● | 1,443,430 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2023, with a weighted average exercise price of $16.58 per share. |
To the extent that any of these options, restricted stock units or warrants are exercised, new options and awards are issued under our equity incentive plans and subsequently exercised or we issue additional common shares or securities convertible into common shares in the future, there may be further dilution to new investors participating in this offering.
9
In the past, we have not declared or paid cash dividends on our common stock, and we do not intend to pay any cash dividends on our common stock. Rather, we intend to retain future earnings, if any, to fund the operation and expansion of our business and for general corporate purposes.
10
On June 28, 2022, we entered into an Amended and Restated Capital on Demand™ Sales Agreement, or the sales agreement, with JonesTrading and B. Riley Securities, pursuant to which we may, from time to time, offer and sell shares of our common stock. Pursuant to this prospectus and the accompanying base prospectus, we may offer and sell shares of our common stock having an aggregate offering price of up to $200,000,000. Sales of our common stock, if any, under this prospectus may be made in sales deemed to be “at the market offerings” as defined in Rule 415 promulgated under the Securities Act.
Each time we wish to issue and sell common stock, we will notify either agent of the number of shares to be issued, the dates on which such sales are anticipated to be made, any minimum price below which sales may not be made and other sales parameters as we deem appropriate. Once we have so instructed an agent, unless that agent declines to accept the terms of the notice, each agent has agreed, subject to the terms and conditions of the sales agreement, to use its commercially reasonable efforts consistent with its normal trading and sales practices to sell such shares up to the amount specified on such terms. We may instruct an agent not to sell shares of common stock if the sales cannot be effected at or above the price designated by us in any such instruction. We or either agent may suspend the offering of shares of common stock being made through the applicable agent under the sales agreement upon proper notice to the other party.
We will pay each agent commissions for its services in acting as agent in the sale of our common stock. Each agent will be entitled to compensation at a commission rate up to 3.0% of the aggregate gross sales price of the shares sold by that agent. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. We have also agreed to reimburse the agents for certain specified expenses, including the fees and disbursements of their legal counsel in an aggregate amount not to exceed $25,000. We estimate that the total expenses for this offering, excluding compensation and reimbursements payable to the agents under the terms of the sales agreement, will be approximately $205,000.
Settlement for sales of common stock will occur on the second business day following the date on which any sales are made or such earlier day as is industry practice for regular-way trading, or on some other date that is agreed upon by us and the applicable agent in connection with a particular transaction, in return for payment of the net proceeds to us. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
In connection with the sale of the common stock on our behalf, each agent will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of each agent will be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and contribution to the agents against certain civil liabilities, including liabilities under the Securities Act.
The offering of shares of common stock pursuant to the sales agreement will terminate upon the earliest of (i) the sale of all shares of common stock subject to the sales agreement and (ii) the termination of the sales agreement according to its terms by either agent or us, with respect to the applicable agent.
Our common stock is listed on the NYSE American and trades under the symbol “ATNM.” The transfer agent of our common stock is Securities Transfer Corporation.
JonesTrading, B. Riley Securities and/or their respective affiliates have in the past and may in the future provide various investment banking and other financial services for us for which services they may in the future receive customary fees.
11
The validity of the securities offered by this prospectus will be passed upon by Haynes and Boone, LLP, New York, New York. Duane Morris LLP, New York, New York, is counsel for JonesTrading and B. Riley Securities in connection with this offering.
The consolidated financial statements of Actinium Pharmaceuticals, Inc. as of and for the years ended December 31, 2022 and 2021 appearing in Actinium Pharmaceuticals, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Marcum LLP, as set forth in its report thereon, including therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
Where You Can Find More Information
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, and in accordance therewith file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission. The Securities and Exchange Commission maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the Securities and Exchange Commission. The address of the Securities and Exchange Commission’s website is www.sec.gov.
We make available free of charge on or through our website at www.actiniumpharma.com, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with or otherwise furnish it to the Securities and Exchange Commission.
We have filed with the Securities and Exchange Commission a registration statement under the Securities Act, relating to the offering of these securities. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement for free at www.sec.gov. The registration statement and the documents referred to below under “Incorporation of Certain Information By Reference” are also available on our website, www.actiniumpharma.com.
We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
12
Incorporation of Certain Information by Reference
The Securities and Exchange Commission allows us to “incorporate by reference” the information we have filed with it, which means that we can disclose important information to you by referring you to those documents. The information we incorporate by reference is an important part of this prospectus, and later information that we file with the Securities and Exchange Commission will automatically update and supersede this information. We incorporate by reference the documents listed below and any future documents (excluding information furnished pursuant to Items 2.02 and 7.01 of Form 8-K) we file with the Securities and Exchange Commission pursuant to Sections l3(a), l3(c), 14 or l5(d) of the Securities Exchange Act of 1934, as amended, subsequent to the date of this prospectus and prior to the termination of the offering:
| ● | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the Securities and Exchange Commission on March 31, 2023; |
| ● | Our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2023, filed with the Securities and Exchange Commission on May 15, 2023; and |
| ● | The description of the Company’s common stock and warrants contained in Exhibit 4.15 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the Securities and Exchange Commission on March 31, 2021, including any amendments thereto or reports filed for the purposes of updating this description. |
All filings filed by us pursuant to the Securities Exchange Act of 1934, as amended, after the date of the initial filing of this registration statement and prior to the effectiveness of such registration statement (excluding information furnished pursuant to Items 2.02 and 7.01 of Form 8-K) shall also be deemed to be incorporated by reference into the prospectus.
You should rely only on the information incorporated by reference or provided in this prospectus. We have not authorized anyone else to provide you with different information. Any statement contained in a document incorporated by reference into this prospectus will be deemed to be modified or superseded for the purposes of this prospectus to the extent that a later statement contained in this prospectus or in any other document incorporated by reference into this prospectus modifies or supersedes the earlier statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date of this prospectus or the date of the documents incorporated by reference in this prospectus.
We will provide without charge to each person to whom a copy of this prospectus is delivered, upon written or oral request, a copy of any or all of the reports or documents that have been incorporated by reference in this prospectus but not delivered with this prospectus (other than an exhibit to these filings, unless we have specifically incorporated that exhibit by reference in this prospectus). Any such request should be addressed to us at:
Actinium Pharmaceuticals, Inc.
Attn: Steve O’Loughlin
100 Park Ave., 23rd Floor
New York, NY 10017
(646) 677-3870
You may also access the documents incorporated by reference in this prospectus through our website at www.actiniumpharma.com. Except for the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated in this prospectus or the registration statement of which it forms a part.
13
$200,000,000

COMMON STOCK
PROSPECTUS
JonesTrading B. Riley Securities
The date of this prospectus is , 2023.
Part II
Information Not Required in Prospectus
| Item | 14. Other Expenses of Issuance and Distribution. |
The fees and expenses payable by us in connection with this registration statement are estimated as follows:
| Securities and Exchange Commission Registration Fee | $ | 10,350 | ||
| FINRA fee | 14,600 | |||
| Accounting Fees and Expenses | 25,000 | |||
| Legal Fees and Expenses | 150,000 | |||
| Miscellaneous Fees and Expenses | 5,000 | |||
| Total | $ | 204,950 |
| Item | 15. Indemnification of Directors and Officers. |
Section 145 of the General Corporation Law of the State of Delaware provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the General Corporation Law of the State of Delaware, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract. Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.
We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the General Corporation Law of the State of Delaware would permit indemnification.
II-1
Item 16. Exhibits.
| * | To be filed, if necessary, by an amendment to the registration statement or incorporated by reference to a Current Report on Form 8-K filed in connection with an underwritten offering of the shares offered hereunder. |
| ** | Filed herewith. |
II-2
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (a) | (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that the undertakings set forth in paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| (i) | If the registrant is relying on Rule 430B (§230.430B of this chapter): |
| (A) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| (B) | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
| (ii) | If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
II-3
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (c) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
| (d) | The undersigned registrant hereby undertakes that: |
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on August 11, 2023.
|
|
ACTINIUM PHARMACEUTICALS, INC. | |
| By: | /s/ Sandesh Seth | |
| Name: | Sandesh Seth | |
| Title: | Chairman and Chief Executive Officer | |
| By: | /s/ Steve O’Loughlin | |
| Name: | Steve O’Loughlin | |
| Title: | Chief Financial Officer | |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints each of Sandesh Seth and Steve O’Loughlin, severally, acting alone and without the other, his or her true and lawful attorney-in-fact and agent, each with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Sandesh Seth | Chairman and Chief Executive Officer and Director | August 11, 2023 | ||
| Sandesh Seth | (principal executive officer) | |||
| /s/ Steve O’Loughlin | Chief Financial Officer | August 11, 2023 | ||
| Steve O’Loughlin | (principal financial and accounting officer) | |||
| /s/ Jeffrey Chell | Director | August 11, 2023 | ||
| Jeffrey Chell | ||||
| /s/ David Nicholson | Director | August 11, 2023 | ||
| David Nicholson | ||||
| /s/ Richard I. Steinhart | Director | August 11, 2023 | ||
| Richard I. Steinhart | ||||
| /s/ Ajit J. Shetty | Director | August 11, 2023 | ||
| Ajit J. Shetty |
II-5