
Exhibit 99.2

ATNM: NYSE AMERICAN February 18, 2022 Iomab - B SIERRA Trial Phase 3 Results

Disclaimer and Safe Harbor The information presented herein contains express and implied forward - looking statements regarding the current intentions, expectations, estimates, opinions and beliefs of Actinium Pharmaceuticals, Inc . (“Actinium”) that are not historical facts . These forward - looking statements include statements regarding Actinium’s expectations for its product candidates (including their therapeutic and commercial potential, anticipated future development activities, anticipated timing of development activities, including initiation of clinical trials and presentations of clinical data and the indications Actinium and its collaborators plan to pursue), future results of operations and financial position, business strategy, strategic collaborations, any royalty or milestone payments and Actinium’s ability to obtain and maintain intellectual property protection for its product candidates . Such forward - looking statements may be identified by words such as “believes”, “may”, “will”, “expects”, “endeavors”, “anticipates”, “intends”, “plans”, “estimates”, “projects”, “should”, “objective” and variations of such words and similar words . These statements are based on management’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium’s products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the “SEC”), including without limitation its most recent annual report on Form 10 - K, subsequent quarterly reports on Forms 10 - Q and Forms 8 - K, each as amended and supplemented from time to time . Any forward - looking statements that Actinium makes in this presentation speak only as of the date of this presentation . Except as required by law, Actinium assumes no obligation to update its forward - looking statements whether as a result of new information, future events or otherwise, after the date hereof . Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by Actinium or any director, employee, agent, or adviser of Actinium . This presentation does not purport to be all - inclusive or to contain all of the information you may desire . The content of this presentation is subject to copyright, which will be asserted by Actinium, and no part of this presentation may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without prior permission in writing from Actinium . 2

Sandesh Seth Chairman & CEO Dr. Madhuri Vusirikala VP, Clinical Development, BMT & Cellular Therapy Dr. Avinash Desai Chief Medical Officer Caroline Yarbrough Chief Commercial Officer Today’s Speakers & Agenda 3 Introduction and Closing Remarks Iomab - B Phase 3 SIERRA Results Significance of SIERRA Results and Next Steps Iomab - B Market Positioning and Opportunity
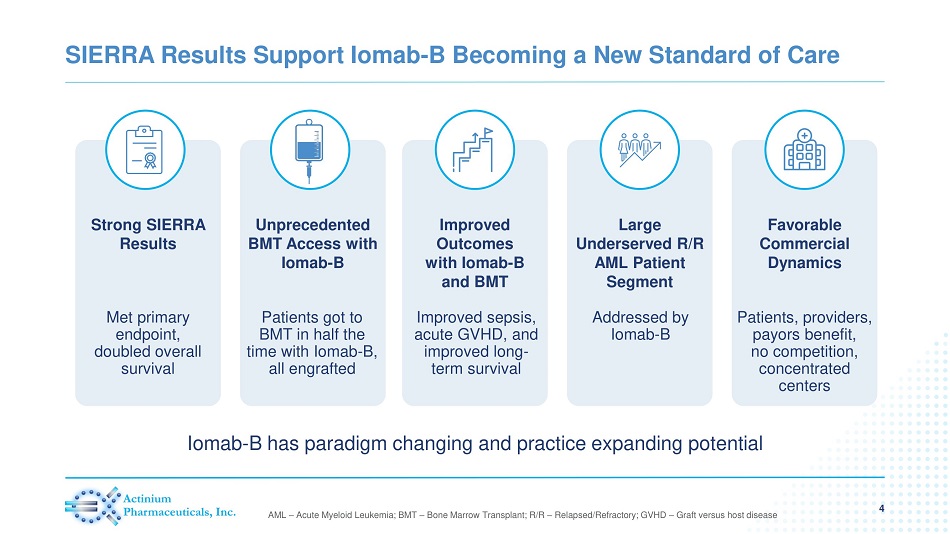
SIERRA Results Support Iomab - B Becoming a New Standard of Care 4 AML – Acute Myeloid Leukemia; BMT – Bone Marrow Transplant; R/R – Relapsed/Refractory; GVHD – Graft versus host disease Unprecedented BMT Access with Iomab - B Large Underserved R/R AML Patient Segment Improved Outcomes with Iomab - B and BMT Strong SIERRA Results Favorable Commercial Dynamics Iomab - B has paradigm changing and practice expanding potential Met primary endpoint, doubled overall survival Patients got to BMT in half the time with Iomab - B, all engrafted Improved sepsis, acute GVHD, and improved long - term survival Addressed by Iomab - B Patients, providers, payors benefit, no competition, concentrated centers

Dr. Madhuri Vusirikala, VP, Clinical Development BMT & Cell Therapy 5 • Joined Actinium in October 2022 as Vice President, Clinical Development, Transplant & Cellular Therapy • Over 20 years of clinical experience specializing in adult allogeneic bone marrow transplant • Most recently, Director of the Allogeneic Stem Cell Transplant Program at University of Texas – Southwestern (UTSW) and Professor of Medicine in the Division of Hematology and Oncology • Serves on several national committees including the National Comprehensive Cancer Network (NCCN) panels for Hematopoietic Stem Cell Transplantation and Acute Lymphoblastic Leukemia, BMT Infonet, and the MDS/Aplastic Anemia Foundation • Fellowship in Bone Marrow Transplant at Vanderbilt University • Fellowship in Hematology - Oncology at the University of Pittsburgh • Residency at SUNY Syracuse • Medical Training at Lady Hardinge College (India)
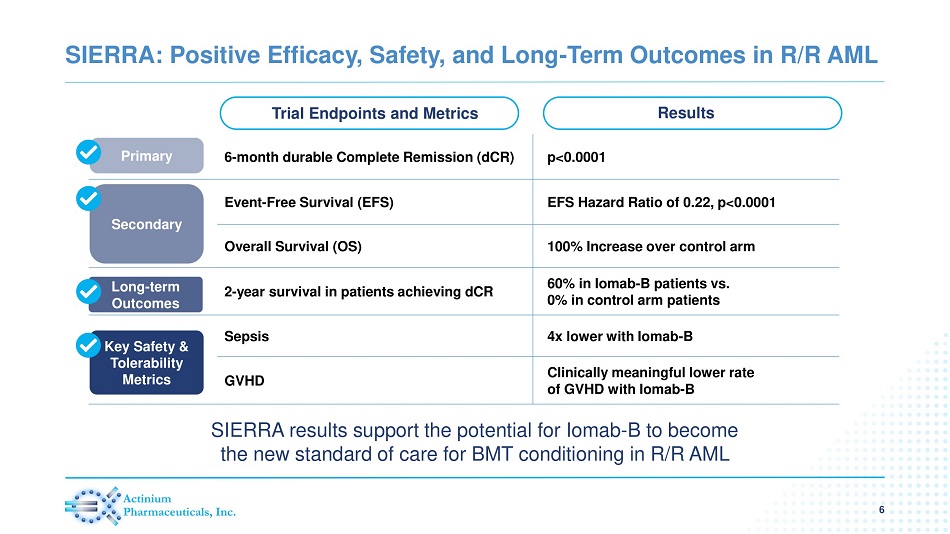
SIERRA: Positive Efficacy, Safety, and Long - Term Outcomes in R/R AML 6 Trial Endpoints and Metrics Results 6 - month durable Complete Remission (dCR) p <0.0001 Event - Free Survival (EFS) EFS Hazard Ratio of 0.22, p<0.0001 Overall Survival (OS) 100% Increase over control arm 2 - year survival in patients achieving dCR 60% in Iomab - B patients vs. 0% in control arm patients Sepsis 4x lower with Iomab - B GVHD Clinically meaningful lower rate of GVHD with Iomab - B Primary Secondary Long - term Outcomes Key Safety & Tolerability Metrics SIERRA results support the potential for Iomab - B to become the new standard of care for BMT conditioning in R/R AML
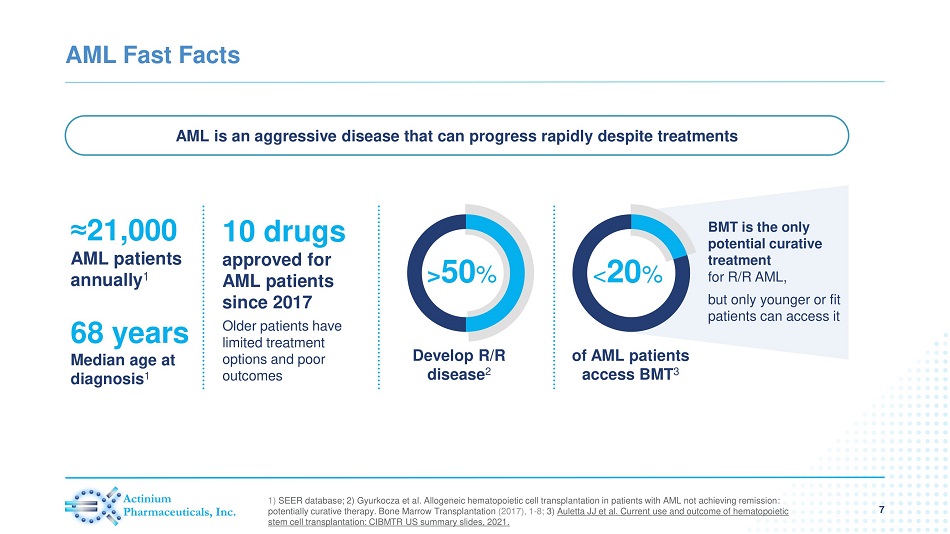
AML Fast Facts 7 ≈ 21,000 AML patients annually 1 AML is an aggressive disease that can progress rapidly despite treatments 68 years Median age at diagnosis 1 Older patients have limited treatment options and poor outcomes Develop R/R disease 2 > 50 % of AML patients access BMT 3 < 20 % BMT is the only potential curative treatment for R/R AML, but only younger or fit patients can access it 1) SEER database; 2) Gyurkocza et al. Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission : potentially curative therapy. Bone Marrow Transplantation (2017), 1 - 8; 3) Auletta JJ et al. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021. 10 drugs approved for AML patients since 2017
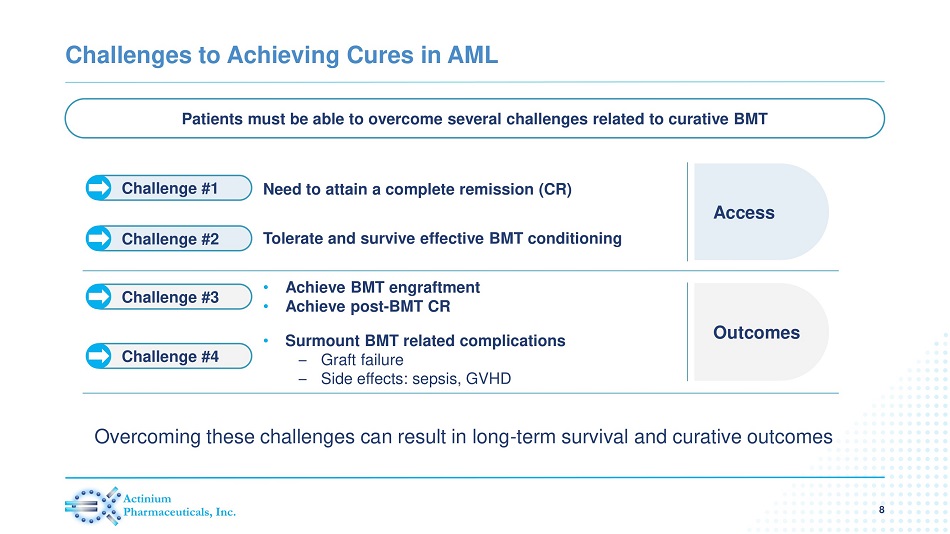
Challenges to Achieving Cures in AML Patients must be able to overcome several challenges related to curative BMT Curative Potential Access Need to attain a complete remission (CR) Tolerate and survive effective BMT conditioning • Achieve BMT engraftment • Achieve post - BMT CR • Surmount BMT related complications – Graft failure – Side effects: sepsis, GVHD Outcomes Challenge #1 Challenge #2 Challenge #3 Challenge #4 Overcoming these challenges can result in long - term survival and curative outcomes 8
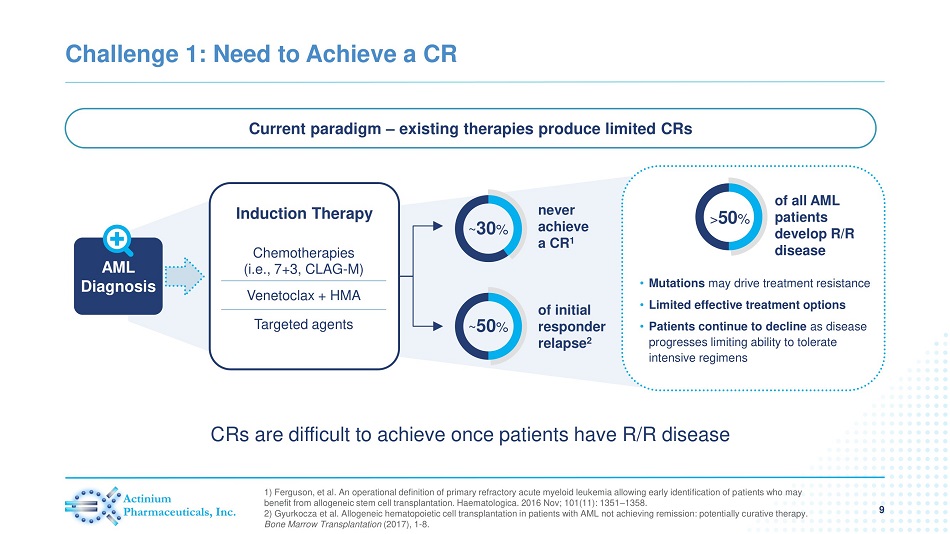
Challenge 1: Need to Achieve a CR 9 1) Ferguson, et al. An operational definition of primary refractory acute myeloid leukemia allowing early identification of p ati ents who may benefit from allogeneic stem cell transplantation. Haematologica. 2016 Nov; 101(11): 1351 – 1358. 2) Gyurkocza et al. Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission: potentially c ura tive therapy. Bone Marrow Transplantation (2017), 1 - 8. Current paradigm – existing therapies produce limited CRs AML Diagnosis of initial responder relapse 2 ~ 50 % never achieve a CR 1 ~ 30 % • Mutations may drive treatment resistance • Limited effective treatment options • Patients continue to decline as disease progresses limiting ability to tolerate intensive regimens of all AML patients develop R/R disease > 50 % Induction Therapy Chemotherapies (i.e., 7+3, CLAG - M) Venetoclax + HMA Targeted agents CRs are difficult to achieve once patients have R/R disease
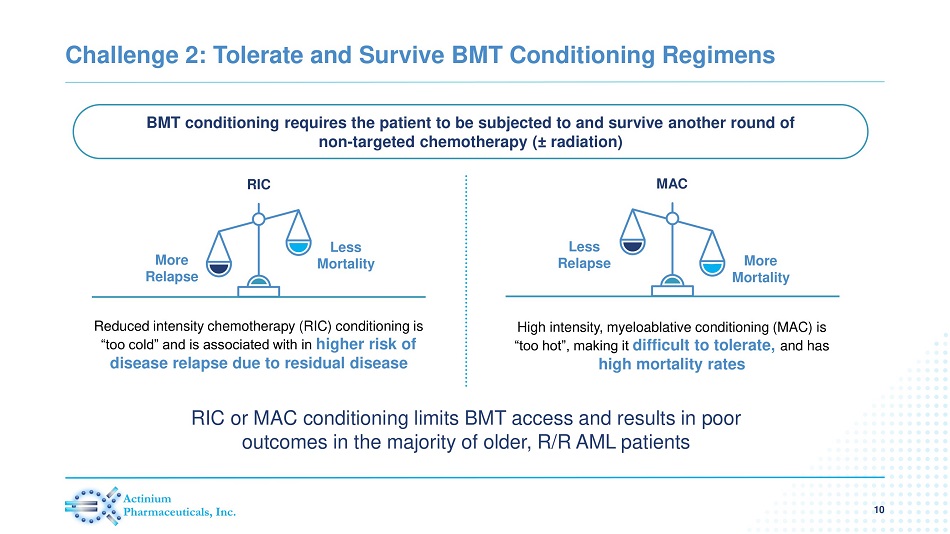
Challenge 2: Tolerate and Survive BMT Conditioning Regimens 10 Reduced intensity chemotherapy (RIC) conditioning is “too cold” and is associated with in higher risk of disease relapse due to residual disease High intensity, myeloablative conditioning (MAC) is “too hot”, making it difficult to tolerate, and has high mortality rates BMT conditioning requires the patient to be subjected to and survive another round of non - targeted chemotherapy ( ± radiation) RIC Less Mortality More Relapse MAC More Mortality Less Relapse RIC or MAC conditioning limits BMT access and results in poor outcomes in the majority of older, R/R AML patients
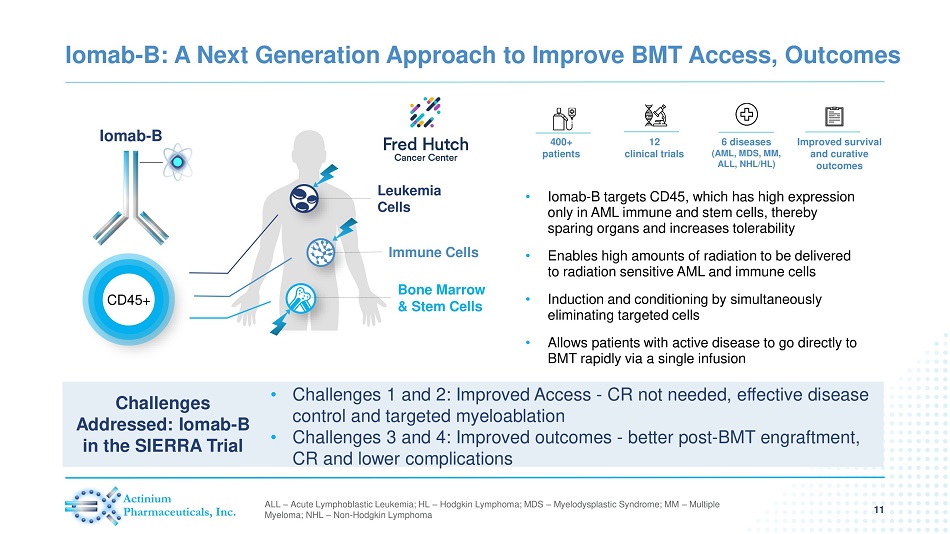
Iomab - B: A Next Generation Approach to Improve BMT Access, Outcomes 11 • Iomab - B targets CD45, which has high expression only in AML immune and stem cells, thereby sparing organs and increases tolerability • Enables high amounts of radiation to be delivered to radiation sensitive AML and immune cells • Induction and conditioning by simultaneously eliminating targeted cells • Allows patients with active disease to go directly to BMT rapidly via a single infusion Iomab - B CD45+ Leukemia Cells Immune Cells Bone Marrow & Stem Cells 400+ patients 12 clinical trials 6 diseases (AML, MDS, MM, ALL, NHL/HL) Improved survival and curative outcomes ALL – Acute Lymphoblastic Leukemia; HL – Hodgkin Lymphoma; MDS – Myelodysplastic Syndrome; MM – Multiple Myeloma; NHL – Non - Hodgkin Lymphoma • Challenges 1 and 2: Improved Access - CR not needed, effective disease control and targeted myeloablation • Challenges 3 and 4: Improved outcomes - better post - BMT engraftment, CR and lower complications Challenges Addressed: Iomab - B in the SIERRA Trial
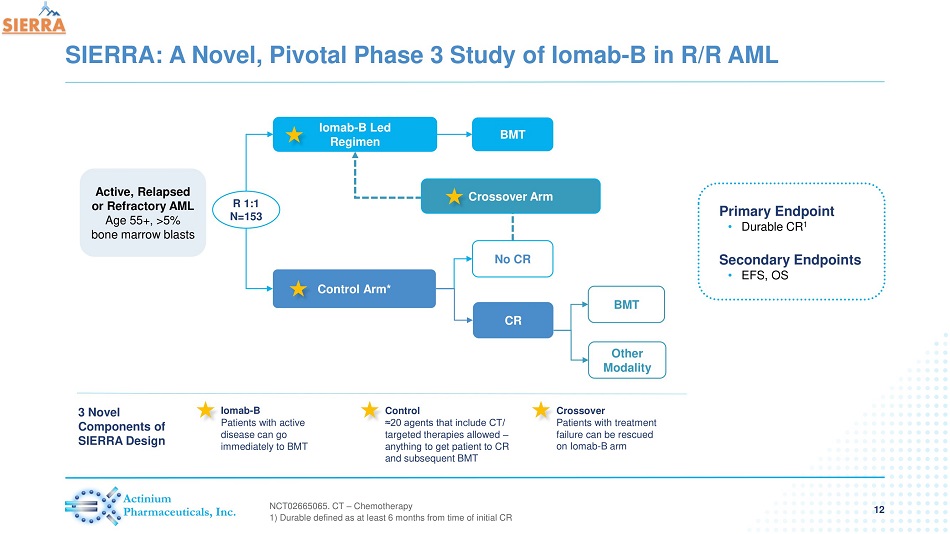
SIERRA: A Novel, Pivotal Phase 3 Study of Iomab - B in R/R AML 12 Primary Endpoint • Durable CR 1 Secondary Endpoints • EFS, OS Iomab - B Led Regimen Control Arm* BMT CR Other Modality No CR BMT Crossover Arm R 1:1 N=153 NCT 02665065 . CT – Chemotherapy 1) Durable defined as at least 6 months from time of initial CR Iomab - B Patients with active disease can go immediately to BMT Control ≈20 agents that include CT/ targeted therapies allowed – anything to get patient to CR and subsequent BMT Crossover Patients with treatment failure can be rescued on Iomab - B arm 3 Novel Components of SIERRA Design Active, Relapsed or Refractory AML Age 55+, >5% bone marrow blasts
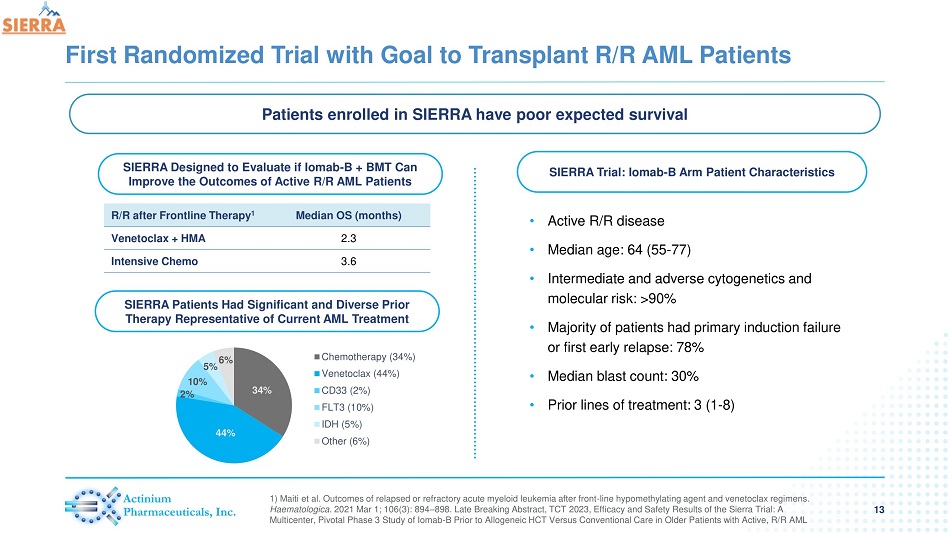
First Randomized Trial with Goal to Transplant R/R AML Patients 13 1) Maiti et al. Outcomes of relapsed or refractory acute myeloid leukemia after front - line hypomethylating agent and venetoclax regimens . Haematologica . 2021 Mar 1; 106(3): 894 – 898. Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Iomab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/ R AML Patients enrolled in SIERRA have poor expected survival SIERRA Trial: Iomab - B Arm Patient Characteristics • Active R/R disease • Median age: 64 (55 - 77) • Intermediate and adverse cytogenetics and molecular risk: >90% • Majority of patients had primary induction failure or first early relapse: 78% • Median blast count: 30% • Prior lines of treatment: 3 (1 - 8) SIERRA Designed to Evaluate if Iomab - B + BMT Can Improve the Outcomes of Active R/R AML Patients R/R after Frontline Therapy 1 Median OS (months) Venetoclax + HMA 2.3 Intensive Chemo 3.6 34% 44% 2% 10% 5% 6% Chemotherapy (34%) Venetoclax (44%) CD33 (2%) FLT3 (10%) IDH (5%) Other (6%) SIERRA Patients Had Significant and Diverse Prior Therapy Representative of Current AML Treatment
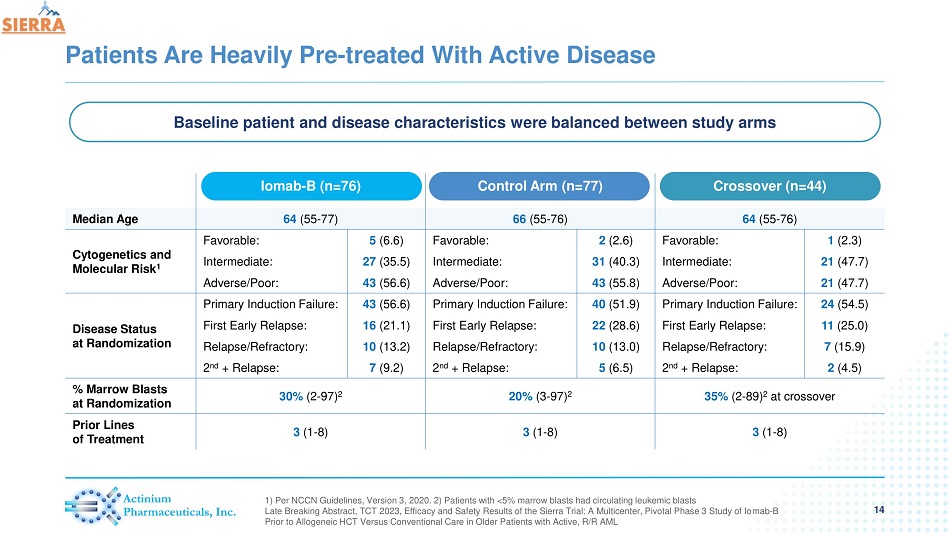
Patients Are Heavily Pre - treated With Active Disease 14 1) Per NCCN Guidelines, Version 3, 2020. 2) Patients with <5% marrow blasts had circulating leukemic blasts Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML Iomab - B (n=76) Control Arm (n=77) Crossover (n=44) Median Age 64 (55 - 77) 66 (55 - 76) 64 (55 - 76) Cytogenetics and Molecular Risk 1 Favorable: 5 (6.6) Favorable: 2 (2.6) Favorable: 1 (2.3) Intermediate: 27 (35.5) Intermediate: 31 (40.3) Intermediate: 21 (47.7) Adverse/Poor: 43 (56.6) Adverse/Poor: 43 (55.8) Adverse/Poor: 21 (47.7) Disease Status at Randomization Primary Induction Failure: 43 (56.6) Primary Induction Failure: 40 (51.9) Primary Induction Failure: 24 (54.5) First Early Relapse: 16 (21.1) First Early Relapse: 22 (28.6) First Early Relapse: 11 (25.0) Relapse/Refractory: 10 (13.2) Relapse/Refractory: 10 (13.0) Relapse/Refractory: 7 (15.9) 2 nd + Relapse: 7 (9.2) 2 nd + Relapse: 5 (6.5) 2 nd + Relapse: 2 (4.5) % Marrow Blasts at Randomization 30% (2 - 97) 2 20% (3 - 97) 2 35% (2 - 89 ) 2 at crossover Prior Lines of Treatment 3 (1 - 8) 3 (1 - 8) 3 (1 - 8) Baseline patient and disease characteristics were balanced between study arms
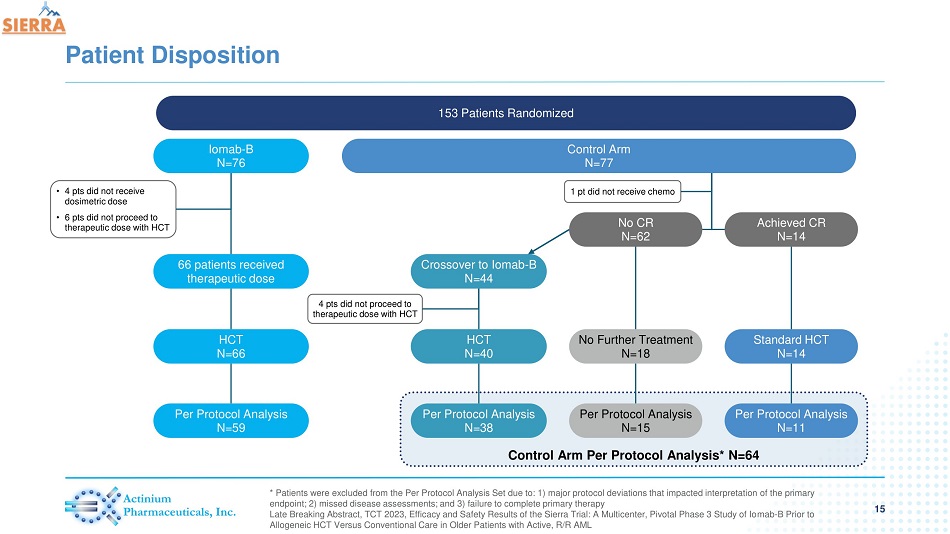
Control Arm Per Protocol Analysis* N=64 Patient Disposition * Patients were excluded from the Per Protocol Analysis Set due to: 1) major protocol deviations that impacted interpretation of the primary endpoint; 2) missed disease assessments; and 3) failure to complete primary therapy Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML 153 Patients Randomized Control Arm N=77 Iomab - B N=76 66 patients received therapeutic dose HCT N=66 Per Protocol Analysis N=59 • 4 pts did not receive dosimetric dose • 6 pts did not proceed to therapeutic dose with HCT Crossover to Iomab - B N=44 HCT N=40 Per Protocol Analysis N=38 No CR N=62 Achieved CR N=14 1 pt did not receive chemo 4 pts did not proceed to therapeutic dose with HCT No Further Treatment N=18 Per Protocol Analysis N=15 Standard HCT N=14 Per Protocol Analysis N=11 15
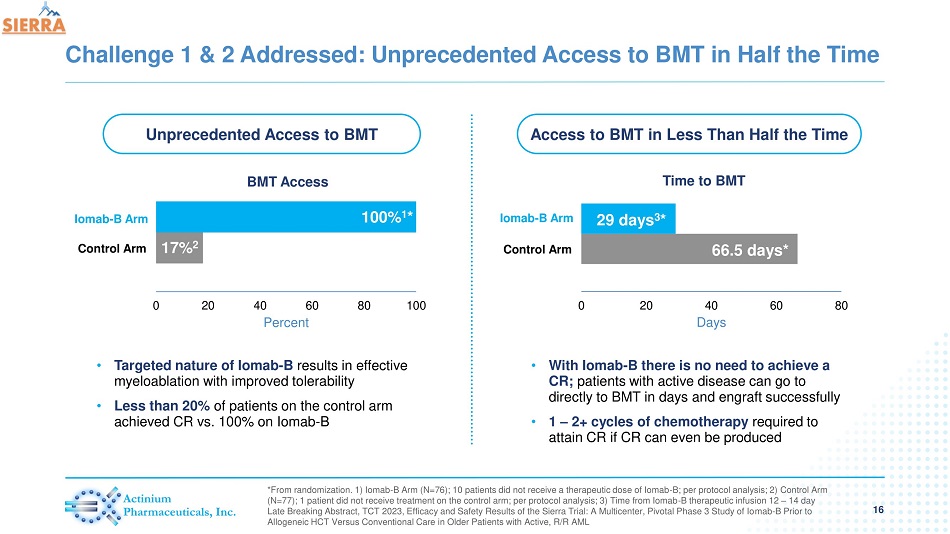
Challenge 1 & 2 Addressed: Unprecedented Access to BMT in Half the Time 16 *From randomization. 1) Iomab - B Arm (N=76); 10 patients did not receive a therapeutic dose of Iomab - B; per protocol analysis; 2) Control Arm (N=77); 1 patient did not receive treatment on the control arm; per protocol analysis; 3) Time from Iomab - B therapeutic infusion 12 – 14 day Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML Iomab - B Arm 66.5 days* 29 days 3 * 0 20 40 60 80 Days Time to BMT 17% 2 100 % 1 * 0 20 40 60 80 100 Percent BMT Access Control Arm Iomab - B Arm Control Arm Unprecedented Access to BMT Access to BMT in Less Than Half the Time • Targeted nature of Iomab - B results in effective myeloablation with improved tolerability • Less than 20% of patients on the control arm achieved CR vs. 100% on Iomab - B • With Iomab - B there is no need to achieve a CR; patients with active disease can go to directly to BMT in days and engraft successfully • 1 – 2+ cycles of chemotherapy required to attain CR if CR can even be produced
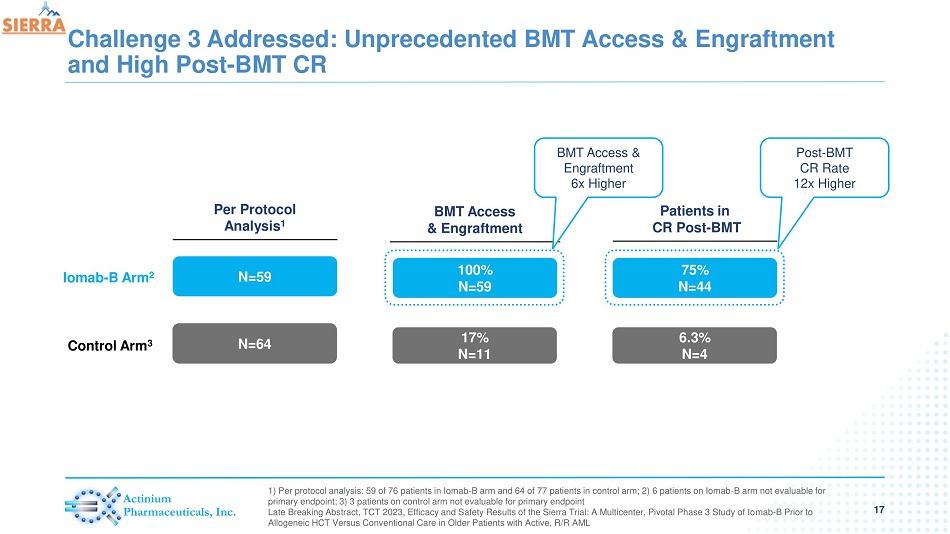
Challenge 3 Addressed: Unprecedented BMT Access & Engraftment and High Post - BMT CR 17 1) Per protocol analysis: 59 of 76 patients in Iomab - B arm and 64 of 77 patients in control arm; 2) 6 patients on Iomab - B arm no t evaluable for primary endpoint; 3) 3 patients on control arm not evaluable for primary endpoint Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML Patients in CR Post - BMT N=59 N=64 Per Protocol Analysis 1 75% N=44 6.3% N=4 BMT Access & Engraftment 100% N=59 17% N=11 Iomab - B Arm 2 Control Arm 3 BMT Access & Engraftment 6 x Higher Post - BMT CR Rate 12x Higher
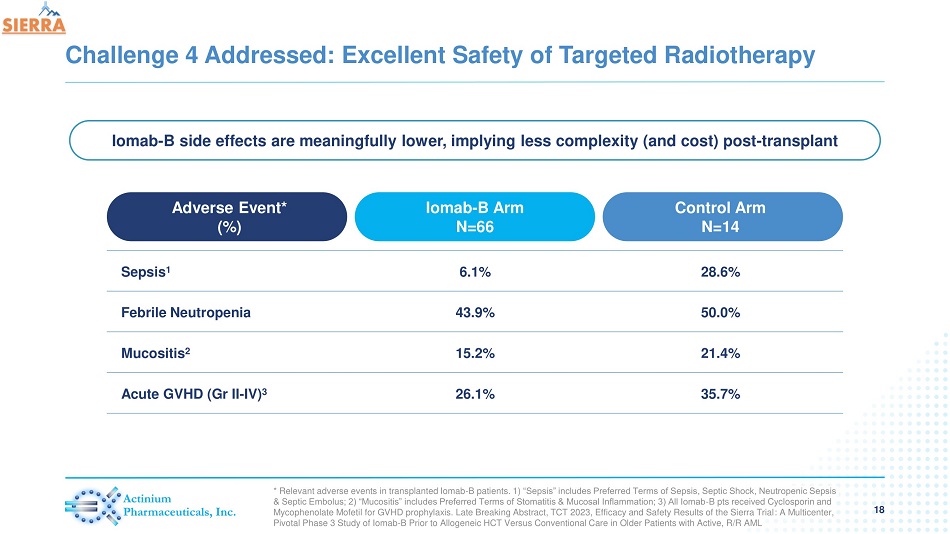
Challenge 4 Addressed: Excellent Safety of Targeted Radiotherapy 18 Adverse Event* (%) Iomab - B Arm N=66 Control Arm N=14 Sepsis 1 6.1% 28.6% Febrile Neutropenia 43.9% 50.0% Mucositis 2 15.2% 21.4% Acute GVHD (Gr II - IV) 3 26.1% 35.7% * Relevant adverse events in transplanted Iomab - B patients. 1) “Sepsis” includes Preferred Terms of Sepsis, Septic Shock, Neutropenic Sepsis & Septic Embolus; 2) “Mucositis” includes Preferred Terms of Stomatitis & Mucosal Inflammation; 3) All Iomab - B pts received Cycl osporin and Mycophenolate Mofetil for GVHD prophylaxis. Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial : A Multicenter, Pivotal Phase 3 Study of Iomab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML Iomab - B side effects are meaningfully lower, implying less complexity (and cost) post - transplant
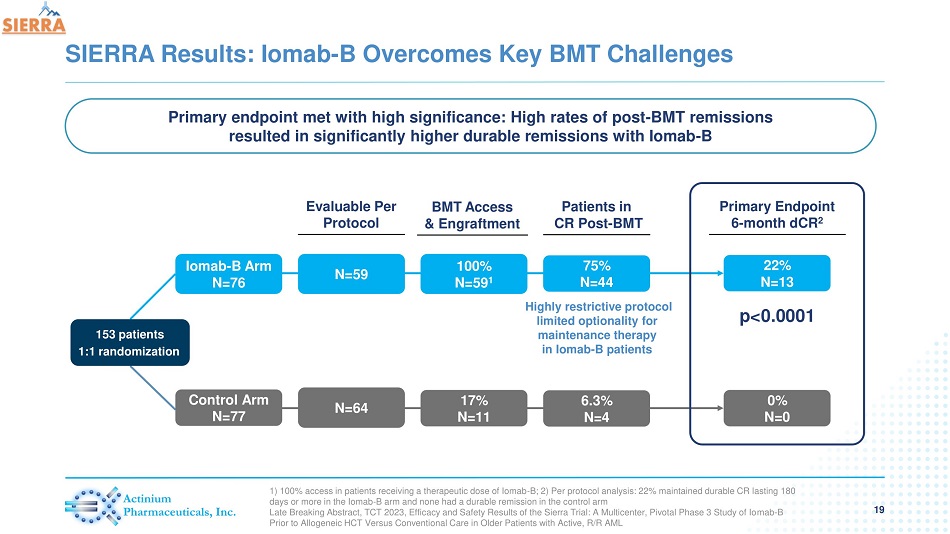
SIERRA Results: Iomab - B Overcomes Key BMT Challenges 19 1) 100% access in patients receiving a therapeutic dose of Iomab - B; 2) Per protocol analysis: 22% maintained durable CR lasting 180 days or more in the Iomab - B arm and none had a durable remission in the control arm Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML Iomab - B Arm N=76 Control Arm N=77 153 patients 1:1 randomization Patients in CR Post - BMT Primary Endpoint 6 - month dCR 2 N=59 N=64 Evaluable Per Protocol 75% N=44 6.3% N=4 22% N=13 0% N=0 BMT Access & Engraftment 100% N=59 1 17% N=11 p<0.0001 Highly restrictive protocol limited optionality for maintenance therapy in Iomab - B patients Primary endpoint met with high significance: High rates of post - BMT remissions resulted in significantly higher durable remissions with Iomab - B
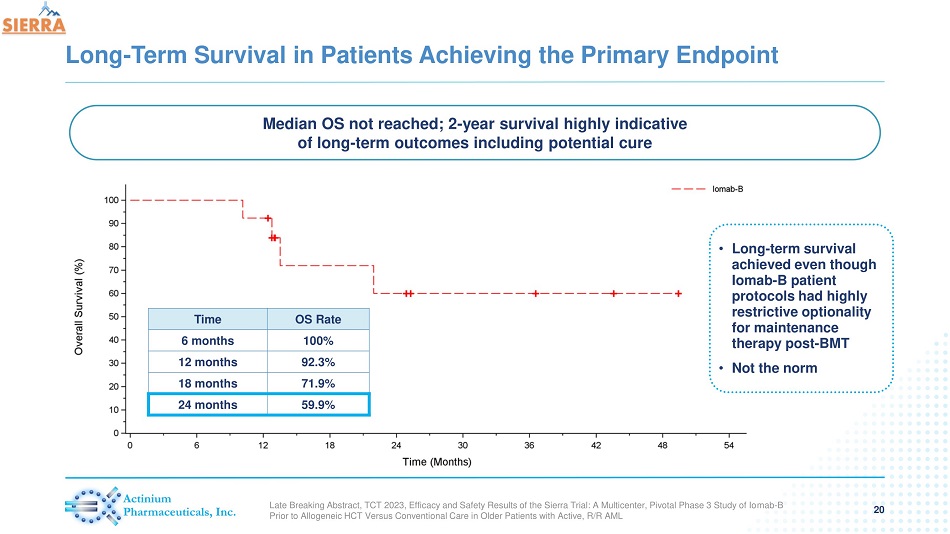
Long - Term Survival in Patients Achieving the Primary Endpoint 20 Time OS Rate 6 months 100% 12 months 92.3% 18 months 71.9% 24 months 59.9% Median OS not reached; 2 - year survival highly indicative of long - term outcomes including potential cure • Long - term survival achieved even though Iomab - B patient protocols had highly restrictive optionality for maintenance therapy post - BMT • Not the norm Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML
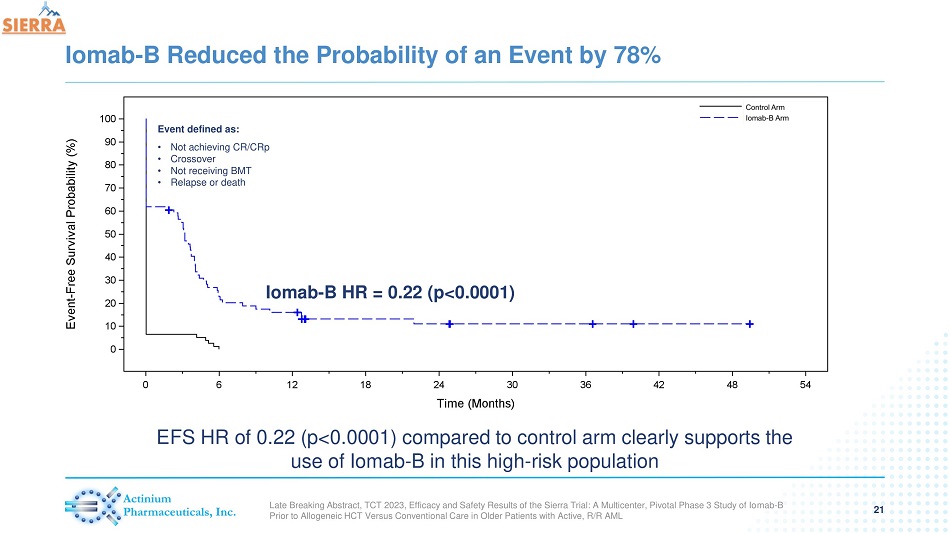
Iomab - B Reduced the Probability of an Event by 78% 21 Iomab - B HR = 0.22 (p<0.0001) Event defined as: • Not achieving CR/CRp • Crossover • Not receiving BMT • Relapse or death EFS HR of 0.22 (p< 0.0001) compared to control arm clearly supports the use of Iomab - B in this high - risk population Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Io mab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML
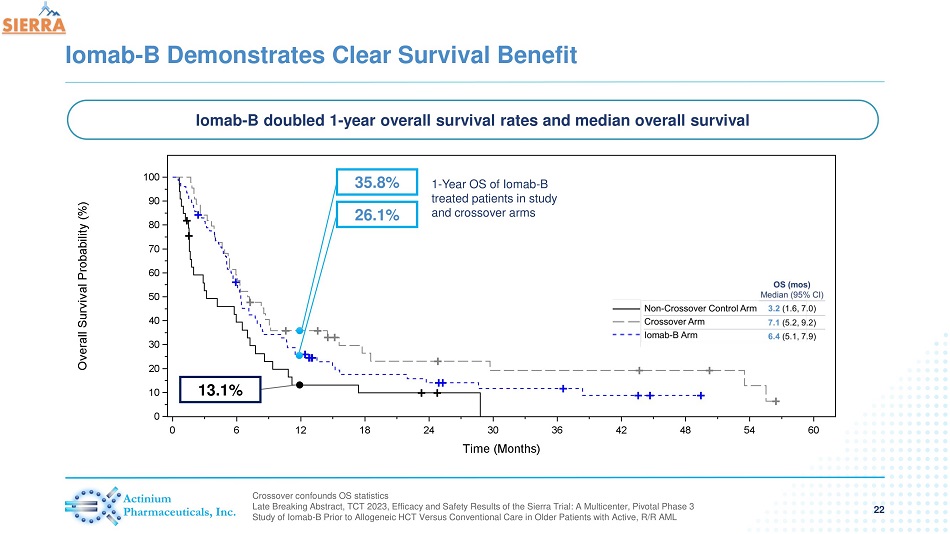
Iomab - B Demonstrates Clear Survival Benefit 22 Crossover confounds OS statistics Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Iomab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML 13.1% 26.1% 35.8% Iomab - B doubled 1 - year overall survival rates and median overall survival 1 - Year OS of Iomab - B treated patients in study and crossover arms
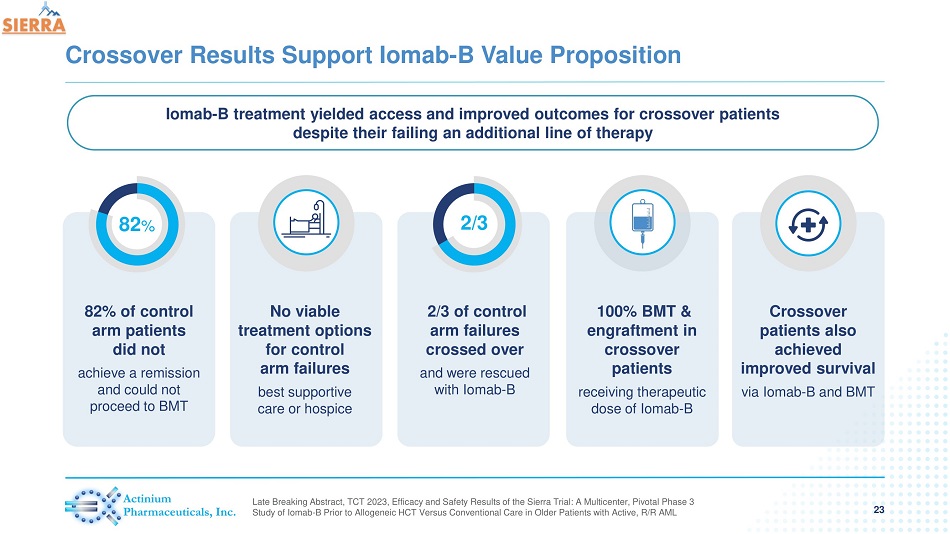
Crossover Results Support Iomab - B Value Proposition 23 82% of control arm patients did not achieve a remission and could not proceed to BMT 82 % No viable treatment options for control arm failures best supportive care or hospice 2/3 of control arm failures crossed over and were rescued with Iomab - B 2/3 100% BMT & engraftment in crossover patients receiving therapeutic dose of Iomab - B Crossover patients also achieved improved survival via Iomab - B and BMT Iomab - B treatment yielded access and improved outcomes for crossover patients despite their failing an additional line of therapy Late Breaking Abstract, TCT 2023, Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Iomab - B Prior to Allogeneic HCT Versus Conventional Care in Older Patients with Active, R/R AML
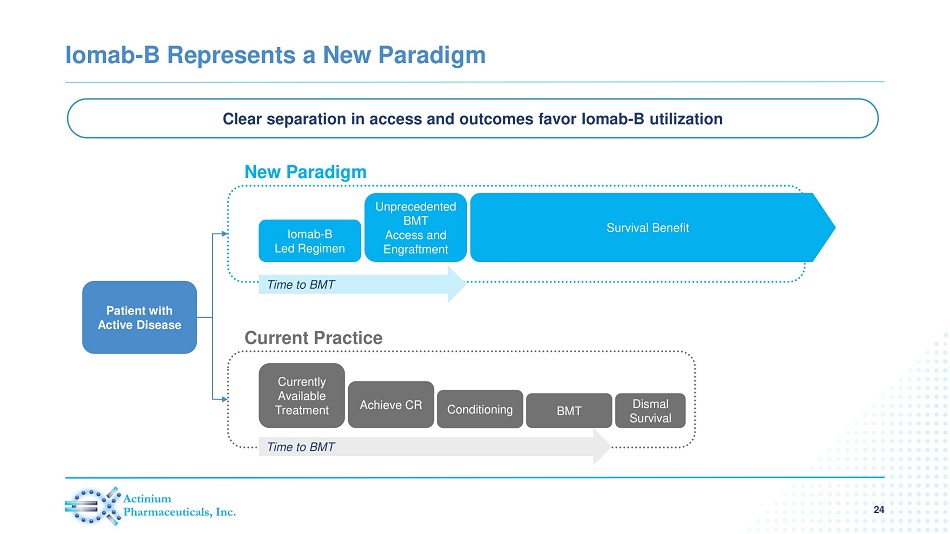
Iomab - B Represents a New Paradigm 24 Clear separation in access and outcomes favor Iomab - B utilization Patient with Active Disease New Paradigm Current Practice Iomab - B Led Regimen Unprecedented BMT Access and Engraftment Time to BMT Currently Available Treatment Achieve CR Conditioning BMT Dismal Survival Time to BMT Survival Benefit

Dr. Avinash Desai, Chief Medical Officer 25 • Joined Actinium in November 2020 and promoted to Chief Medical Officer in 2021 • 27 years of biopharmaceutical experience in clinical development and medical affairs • Joined Actinium from GSK where he was Vice President, Head of U.S. Medical Affairs - Oncology, launching 3 oncology products in 15 months • Previous experience at Janssen Pharmaceuticals (Johnson & Johnson), Eli Lilly & Co. Takeda, Inc. and Sanofi • Contributed to the development and supported multiple blockbuster products including Darzalex and Velcade at Janssen • Participated in multiple successful NDA submissions, launch readiness strategies and execution and life cycle management plans
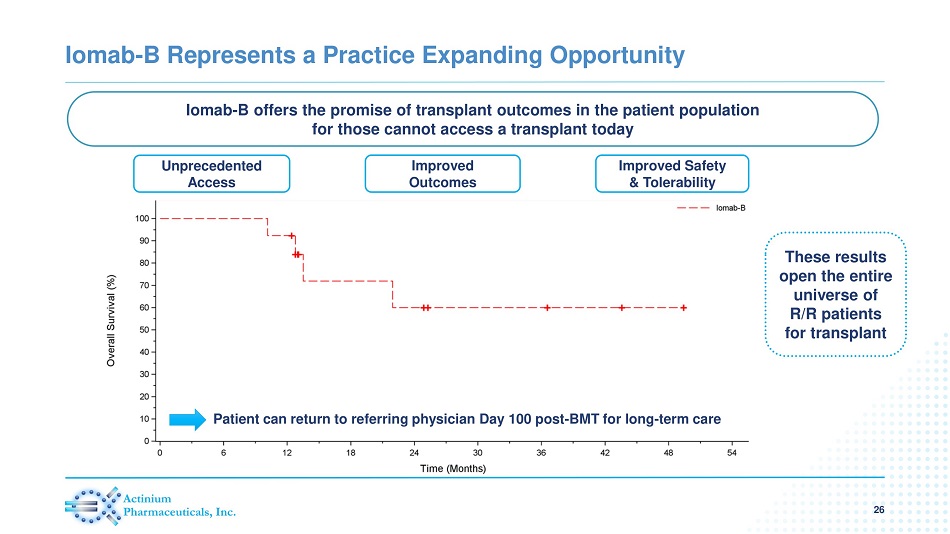
Iomab - B Represents a Practice Expanding Opportunity 26 Improved Safety & Tolerability Improved Outcomes Patient can return to referring physician Day 100 post - BMT for long - term care Unprecedented Access Iomab - B offers the promise of transplant outcomes in the patient population for those cannot access a transplant today These results open the entire universe of R/R patients for transplant
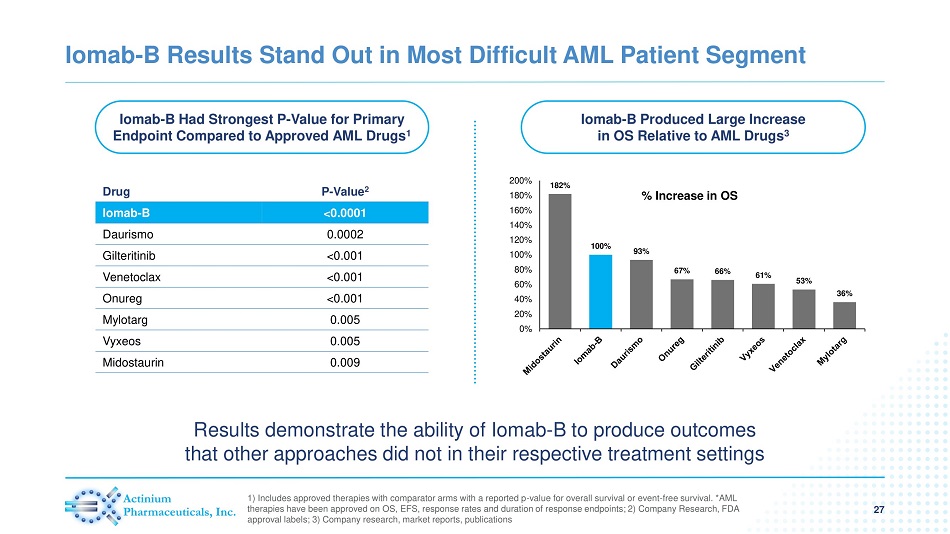
Iomab - B Had Strongest P - Value for Primary Endpoint Compared to Approved AML Drugs 1 Iomab - B Produced Large Increase in OS Relative to AML Drugs 3 Iomab - B Results Stand Out in Most Difficult AML Patient Segment 27 1) Includes approved therapies with comparator arms with a reported p - value for overall survival or event - free survival. *AML therapies have been approved on OS, EFS, response rates and duration of response endpoints; 2) Company Research, FDA approval labels; 3) Company research, market reports, publications % Increase in OS Drug P - Value 2 Iomab - B <0.0001 Daurismo 0.0002 Gilteritinib <0.001 Venetoclax <0.001 Onureg <0.001 Mylotarg 0.005 Vyxeos 0.005 Midostaurin 0.009 182% 100% 93% 67% 66% 61% 53% 36% 0% 20% 40% 60% 80% 100% 120% 140% 160% 180% 200% Results demonstrate the ability of Iomab - B to produce outcomes that other approaches did not in their respective treatment settings
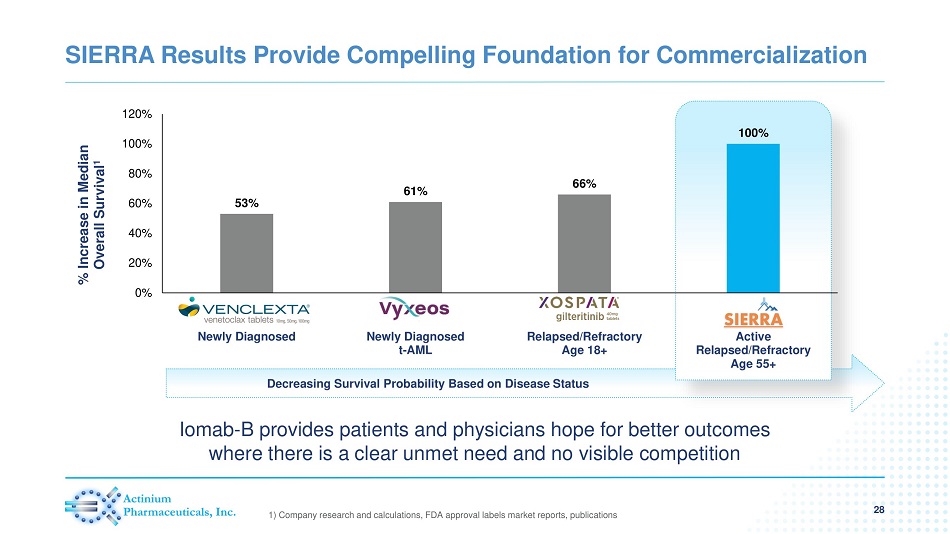
Decreasing Survival Probability Based on Disease Status SIERRA Results Provide Compelling Foundation for Commercialization 28 1) Company research and calculations, FDA approval labels market reports, publications 53% 61% 66% 100% 0% 20% 40% 60% 80% 100% 120% Newly Diagnosed Newly Diagnosed t-AML Relapsed/Refractory Age 18+ Active Relapsed/Refractory Age 55+ % Increase in Median Overall Survival 1 Iomab - B provides patients and physicians hope for better outcomes where there is a clear unmet need and no visible competition
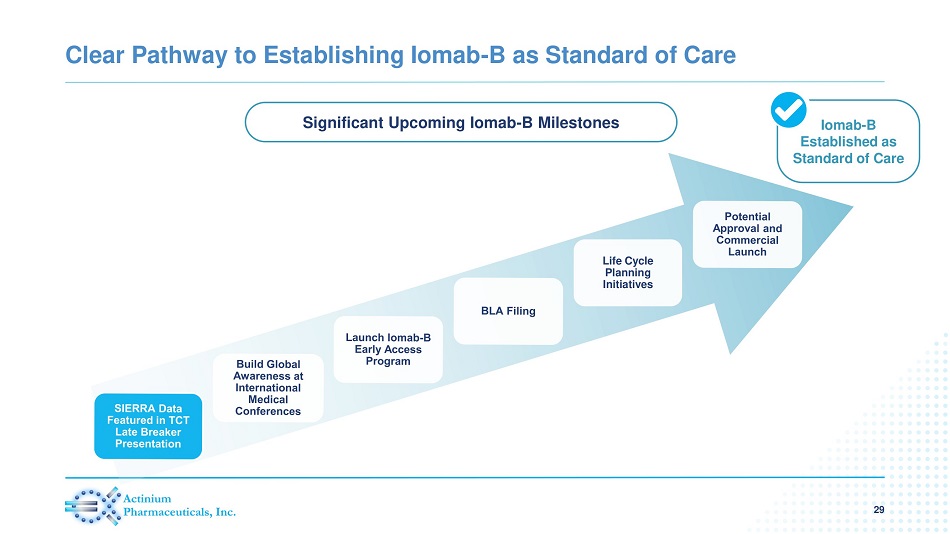
Clear Pathway to Establishing Iomab - B as Standard of Care 29 Iomab - B Established as Standard of Care Life Cycle Planning Initiatives Significant Upcoming Iomab - B Milestones
SIERRA Sets Foundation to Leverage Robust Iomab - B Data • Extensive data and consistent results from more than 400 patients including Fred Hutchinson Cancer Center studies demonstrate high BMT access and engraftment and improved outcomes in patients with MDS, younger AML, ALL, HL/NHL and MM • These indications represent tens of thousands of patients with R/R disease having similar unmet needs to the AML patients in SIERRA • These data, together with the strong results from the pivotal Phase 3 trial will be leveraged to execute our comprehensive life cycle management strategies to further expand Iomab - B’s role in these variety of malignant and non - malignant hematological disorders • Patients are treated by BMT physicians in the same concentrated, high - volume centers, most of which are SIERRA sites Life cycle management and indication expansion opportunity supported by significant body of data with Iomab - B 400+ patients 12 clinical trials 6 diseases (AML, MDS, MM, ALL, NHL/HL) Improved survival and curative outcomes 30
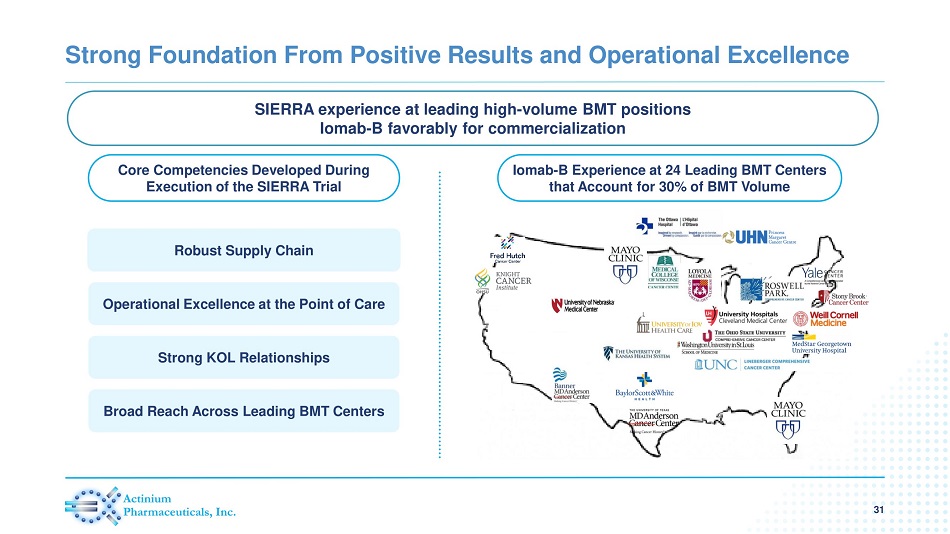
Core Competencies Developed During Execution of the SIERRA Trial Iomab - B Experience at 24 Leading BMT Centers that Account for 30% of BMT Volume Strong Foundation From Positive Results and Operational Excellence 31 Robust Supply Chain Operational Excellence at the Point of Care Strong KOL Relationships Broad Reach Across Leading BMT Centers % Increase in OS SIERRA experience at leading high - volume BMT positions Iomab - B favorably for commercialization

Caroline Yarbrough, Chief Commercial Officer 32 • Joined Actinium in October 2022 with 25 years of BioPharma commercial experience • Most recently, Portfolio General Manager, US Oncology at Novartis, a $1+ Billion product portfolio • Strong hematology experience from leading Novartis Chronic Myelogenous Leukemia (CML) portfolio including Scemblix and Tasigna • Led strategic account management to support the launch of the first approved CAR - T therapy Kymriah • Significant prior experience at GSK, BMS, Viropharma and Merck • Deep understanding of the hematology market, CAR - T/BMT center dynamics, and experience with strategic planning and growing businesses in multiple oncology disease areas
Iomab - B Has a Paradigm Changing Profile Iomab - B Checks All Boxes to Become New Standard of Care Practice Expanding Potential that Will Allow BMT Physicians to Transplant More Patients Unprecedented Access to BMT Excellent Safety and Tolerability Meaningful Survival Benefit • Changes the paradigm by enabling R/R patients to go to BMT without physicians having to learn a “new trick” • Continue to practice BMT the same way, but for more patients • Opens up 50% of AML patients that are currently not treatable to potentially curative BMT • Potential to produce long - term outcomes for patients who currently have 2 - 3 - month survival • Physician regains patients after BMT and gets to see their patients for long - term follow - up care Iomab - B can address a high unmet need in AML while bringing value to patients and physicians 33
Highly Favorable Dynamics Support Iomab - B Commercial Prospects Patients • Unprecedented access to potentially curative BMT in AML with favorable safety and tolerability Physicians • Increased opportunity to treat patients that previously had no other treatment options without disruption to current practice • Referring physicians get their patient back post - BMT for follow - up and long - term care Payors • Strong pharmacoeconomic value proposition driven by Iomab - B product profile Competition • No direct or indirect visible competition for the next 5 - 10 years Concentrated Market • Top 50 centers perform 75% of BMTs; concentrated in metropolitan areas • 12 - 15 account managers to cover BMT center footprint , 35 - 50 - person commercial organization 3P’s, 2C’s: Key variables impacting commercial success align favorably toward Iomab - B 34
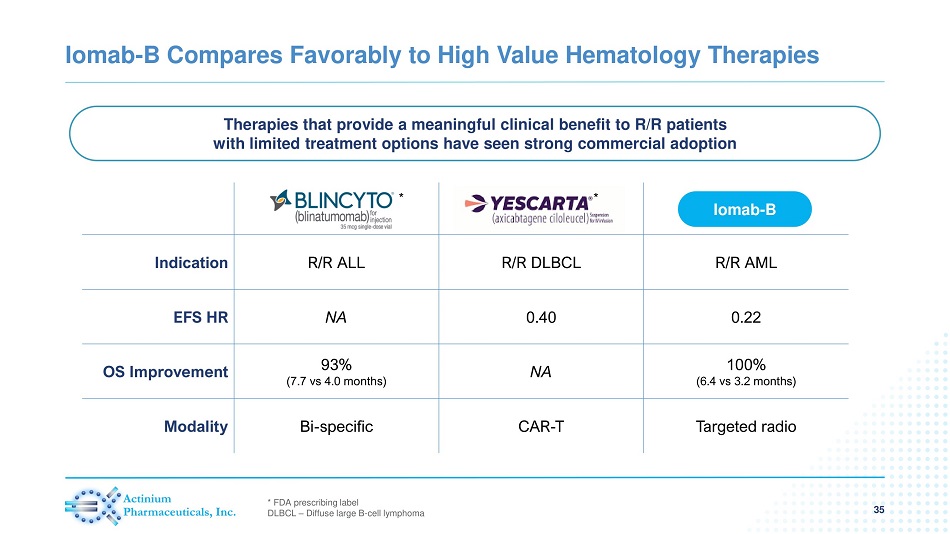
Indication R/R ALL R/R DLBCL R/R AML EFS HR NA 0.40 0.22 OS Improvement 93% (7.7 vs 4.0 months) NA 100% (6.4 vs 3.2 months) Modality Bi - specific CAR - T Targeted radio Iomab - B Compares Favorably to High Value Hematology Therapies 35 Iomab - B Therapies that provide a meaningful clinical benefit to R/R patients with limited treatment options have seen strong commercial adoption * FDA prescribing label DLBCL – Diffuse large B - cell lymphoma * *

Closing Remarks Actinium Pharmaceuticals, Inc.
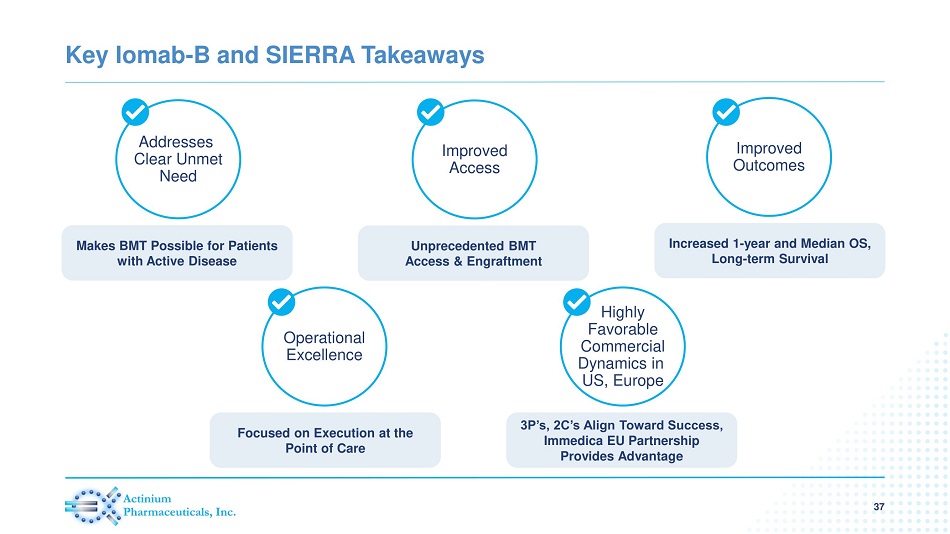
Key Iomab - B and SIERRA Takeaways 37 Addresses Clear Unmet Need Makes BMT Possible for Patients with Active Disease Improved Access Unprecedented BMT Access & Engraftment Improved Outcomes Increased 1 - year and Median OS, Long - term Survival Highly Favorable Commercial Dynamics in US, Europe 3P’s, 2C’s Align Toward Success, Immedica EU Partnership Provides Advantage Operational Excellence Focused on Execution at the Point of Care
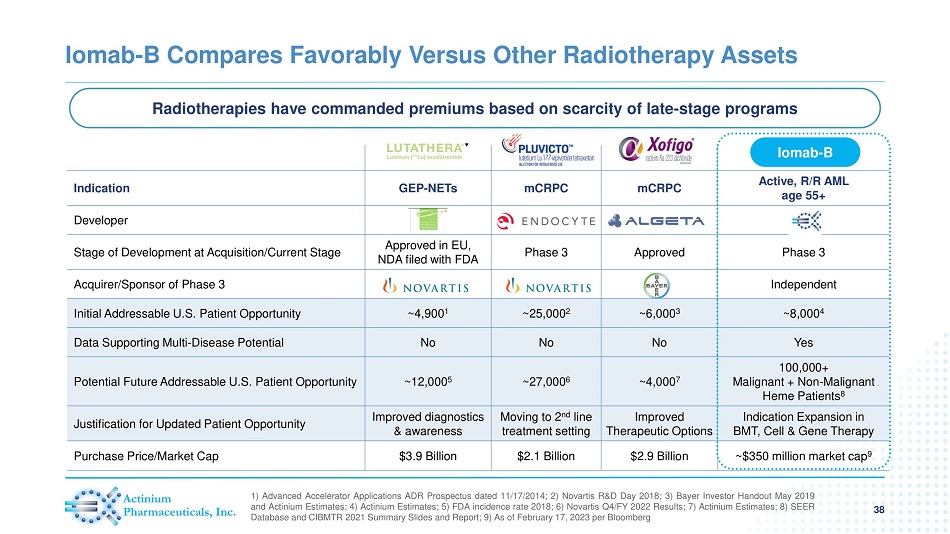
Iomab - B Compares Favorably Versus Other Radiotherapy Assets 38 1 ) Advanced Accelerator Applications ADR Prospectus dated 11 / 17 / 2014 ; 2 ) Novartis R&D Day 2018 ; 3 ) Bayer Investor Handout May 2019 and Actinium Estimates ; 4 ) Actinium Estimates ; 5 ) FDA incidence rate 2018 ; 6 ) Novartis Q 4 /FY 2022 Results ; 7 ) Actinium Estimates ; 8 ) SEER Database and CIBMTR 2021 Summary Slides and Report ; 9 ) As of February 17 , 2023 per Bloomberg Indication GEP - NETs mCRPC mCRPC Active, R/R AML age 55+ Developer Stage of Development at Acquisition/Current Stage Approved in EU, NDA filed with FDA Phase 3 Approved Phase 3 Acquirer/Sponsor of Phase 3 Independent Initial Addressable U.S. Patient Opportunity ~4,900 1 ~25,000 2 ~6,000 3 ~8,000 4 Data Supporting Multi - Disease Potential No No No Yes Potential Future Addressable U.S. Patient Opportunity ~12,000 5 ~27,000 6 ~4,000 7 100,000+ Malignant + Non - Malignant Heme Patients 8 Justification for Updated Patient Opportunity Improved diagnostics & awareness Moving to 2 nd line treatment setting Improved Therapeutic Options Indication Expansion in BMT, Cell & Gene Therapy Purchase Price/Market Cap $3.9 Billion $2.1 Billion $2.9 Billion ~$350 million market cap 9 Iomab - B Radiotherapies have commanded premiums based on scarcity of late - stage programs
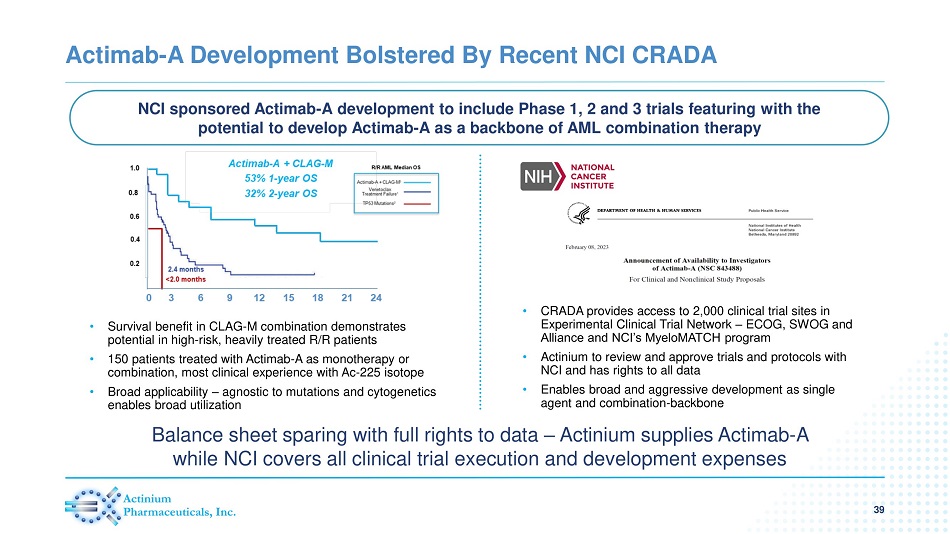
Actimab - A Development Bolstered By Recent NCI CRADA 39 NCI sponsored Actimab - A development to include Phase 1, 2 and 3 trials featuring with the potential to develop Actimab - A as a backbone of AML combination therapy Balance sheet sparing with full rights to data – Actinium supplies Actimab - A while NCI covers all clinical trial execution and development expenses • Survival benefit in CLAG - M combination demonstrates potential in high - risk, heavily treated R/R patients • 150 patients treated with Actimab - A as monotherapy or combination, m ost clinical experience with Ac - 225 isotope • Broad applicability – agnostic to mutations and cytogenetics enables broad utilization • CRADA provides access to 2,000 clinical trial sites in Experimental Clinical Trial Network – ECOG, SWOG and Alliance and NCI’s MyeloMATCH program • Actinium to review and approve trials and protocols with NCI and has rights to all data • Enables broad and aggressive development as single agent and combination - backbone
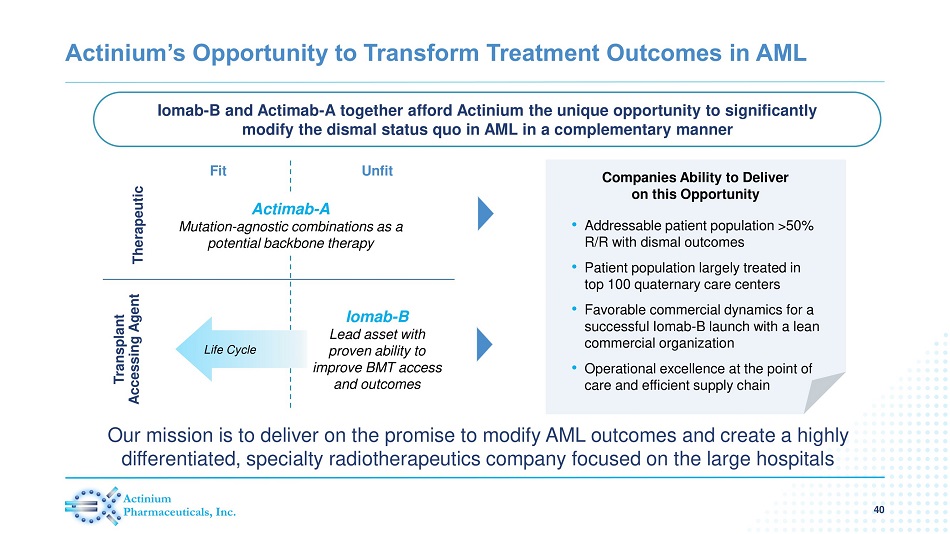
Actinium’s Opportunity to Transform Treatment Outcomes in AML • Addressable patient population >50% R/R with dismal outcomes • Patient population largely treated in top 100 quaternary care centers • Favorable commercial dynamics for a successful Iomab - B launch with a lean commercial organization • Operational excellence at the point of care and efficient supply chain Iomab - B and Actimab - A together afford Actinium the unique opportunity to significantly modify the dismal status quo in AML in a complementary manner 40 Companies Ability to Deliver on this Opportunity Therapeutic Transplant Accessing Agent Fit Unfit Actimab - A Mutation - agnostic combinations as a potential backbone therapy Life Cycle Iomab - B Lead asset with proven ability to improve BMT access and outcomes Our mission is to deliver on the promise to modify AML outcomes and create a highly differentiated, specialty radiotherapeutics company focused on the large hospitals

Q&A Actinium Pharmaceuticals, Inc.

ATNM: NYSE AMERICAN February 18, 2022 Thank you Actinium Pharmaceuticals, Inc.