
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934
Filed by the Registrant ☒ Filed by a Party other than the Registrant ☐
Check the appropriate box:
| ☐ | Preliminary Proxy Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ☐ | Definitive Proxy Statement |
| ☒ | Definitive Additional Materials |
| ☐ | Soliciting Material Pursuant to §240.14a-12 |
Actinium Pharmaceuticals, Inc.
(Name of Registrant as Specified In Its Charter)
N/A
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ | No fee required. | |
| ☐ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |
| 1. |
Title of each class of securities to which transaction applies:
| |
| 2. |
Aggregate number of securities to which transaction applies:
| |
| 3. |
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
| |
| 4. |
Proposed maximum aggregate value of transaction:
| |
| 5. |
Total fee paid:
| |
| ☐ | Fee paid previously with preliminary materials: | |
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing. | |
| 1. |
Amount previously paid:
| |
| 2. |
Form, Schedule or Registration Statement No.:
| |
| 3. |
Filing Party:
| |
| 4. |
Date Filed:
| |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 28, 2020
ACTINIUM PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36374 | 74-2963609 | ||
| (State or other
jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
275 Madison Avenue, 7th Floor, New York, NY 10016
(Address of Principal Executive Offices)
Registrant’s telephone number: (646) 677-3870
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | ATNM | NYSE American |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On October 28, 2020 , Actinium Pharmaceuticals, Inc. (the “Company”) issued a letter to shareholders, which is attached hereto as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
In accordance with General Instruction B.2 of Form 8-K, the information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended, except as shall be expressly set forth by reference in such a filing. Furthermore, the furnishing of information under Item 7.01 of this Current Report on Form 8-K is not intended to constitute a determination by the Company that the information contained herein, including the exhibits hereto, is material or that the dissemination of such information is required by Regulation FD.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number | Description | |
| 99.1 | 2020 Shareholder Letter, dated October 28, 2020 (furnished herewith pursuant to Item 7.01). |
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Actinium Pharmaceuticals, Inc. | |
| Date: October 28, 2020 | /s/ Sandesh Seth |
| Name: Sandesh Seth | |
| Title: Chairman and Chief Executive Officer |
2
Exhibit 99.1

October 2020
Dear Actinium Shareholder:
In 2020 thus far, we have made steady progress with our Iomab-B SIERRA pivotal trial, which is in its final quartile of enrollment, and our Actimab-A combination trials in the Relapsed and Refractory Acute Myeloid Leukemia (R/R AML) setting. We are on track and look forward to data updates related to this progress by year-end; specifically, data from the first 75% of patients from the SIERRA trial, the Ad-Hoc interim analysis, and POC data from our Actimab-A + CLAG-M combination trial. In addition, we have several other milestones across our platform that will be reached in 4Q:2020 and 2021, setting the stage for a potentially transformational future for your company. We approach these milestones with momentum across our clinical pipeline, enhanced R&D capabilities, a strong balance sheet and highly motivated colleagues. We are excited for Actinium’s future, grateful for your support and look forward to creating value for all stakeholders.
Attractive Multi-Product, Multi-Indication Opportunity in R/R AML Emerges in 2020
The progress made this year with Iomab-B and the Actimab-A therapeutic combination trials is exciting and potentially transformational. Taken together these programs can address the still unmet needs of a large segment of patients with R/R AML and potentially change the way the disease is treated. Despite 9 approved therapies for AML, a majority of patients relapse or become refractory at which point treatment options are limited. Bone Marrow Transplant (BMT) remains the only curative treatment option but R/R AML patients typically cannot receive one as they are unable to withstand the highly toxic chemotherapy conditioning agents currently being used. As depicted in the graphic below, the ongoing pivotal Phase 3 SIERRA trial with Iomab-B is the backbone for our R/R AML strategy; enabling elderly, unfit patients to access a BMT with the potential for improved survival. Our Actimab-A combination trials with CLAG-M and venetoclax could be used either as therapeutics or a bridge to transplant in the fit and unfit patient segments. We eagerly await the data events for Iomab-B and the proof of concept data for both Actimab-A combination trials by year-end and in 2021, respectively. Together these results could set the stage for a much larger and attractive opportunity in R/R AML than either drug candidate alone.
Upcoming Data Could Set Stage for Expanded Market Opportunity in R/R AML
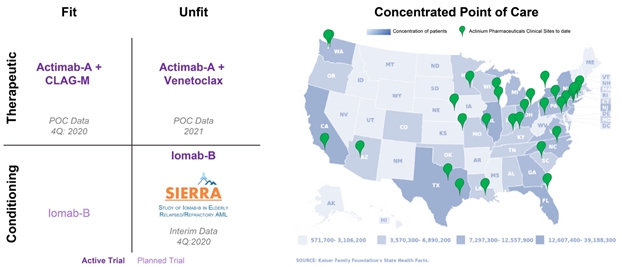
The R/R AML patient population is largely treated in approximately 75-100 tertiary care hospitals, which also conduct the majority of BMT procedures. Leveraging relationships established with the leading AML treatment centers in our clinical trials and our supply chain capabilities, we believe that Iomab-B and Actimab-A could be successfully commercialized into the tertiary care setting and envision building Actinium into a specialty oncology company focused on addressing the R/R AML patient population with multiple products. Our programs are on the cusp of producing data that can help us realize this vision.
2020 – Accomplishments Across All Major Pipeline Initiatives
As we hear of COVID-19 ad nauseum, I am proud to report that Actinium was able to accomplish many of its clinical goals despite clinical sites being severely hampered for several months. Our SIERRA trial was able to maintain enrollment because the trial investigators needed to keep treating very sick R/R AML patients and recognized the demonstrated benefit of Iomab-B from the 50 percent interim analysis. Throughout this period, dedicated teams at Actinium and our clinical sites were able to ensure that our supply chain capabilities were fully operational. Highlighted below are some of the achievements we have made in 2020 and outlook into our major programs.
Targeted Conditioning Pipeline – Value Creating Data Events Within Reach
Iomab-B Program for BMT - Pivotal Phase 3 SIERRA Trial
We note that, despite the late trial stage, investigator interest driven by data updates published at the 50 percent mark has led to several additional sites joining or expected to join the study. This data reported at the Transplantation & Cellular Therapy Conference showed that 100% of patients receiving Iomab-B underwent a BMT and engrafted, the first sign of a successful BMT outcome. Only 18% of control arm patients successfully received a BMT. Additionally, the difference in number of patients potentially evaluable for the primary endpoint, measured by 100-day non-relapse transplant related mortality, has remained consistent at roughly 6x greater for the study arm at the 25% and 50% enrollment updates. With the trial in its final quartile of enrollment, we look forward to data updates from the 75 percent enrollment mark and the interim ad-hoc analysis by year-end. The strong investigator interest in Iomab-B is a barometer for the unmet need and we are hopeful that Iomab-B will be able to provide these patients improved access to a BMT and better outcomes.
Iomab-ACT Program for CAR-T – Proof of Concept Trial with MSK
We are collaborating with Memorial Sloan Kettering Cancer Center (MSK) via an approach validating NIH STTR fast track grant to explore the benefit of using Iomab-ACT, a low dose version of the ARC used in the Iomab-B program, to condition patients before they receive CAR-T therapy. MSK is a leader in the field of cellular therapy and this trial is unique as it will be the first time an ARC has been used for conditioning for any cellular therapy. The CAR-T construct, 19-28z, used in this trial, has proven efficacy in various blood cancers but is not truly viable due to relatively high rates of cytokine release syndrome and neurotoxicity. Iomab-ACT can potentially address these issues by selectively targeting and depleting immune cells implicated in these CAR-T toxicities and creating a better environment for the CAR-T cells to expand and persist in order to attack the patient’s cancer and potentially have lower side effects and higher and more durable responses. We look forward to proof of concept data from this trial in 2021. There is an attractive and potentially large opportunity for the Iomab-ACT program to become the universal conditioning regimen for CAR-T based therapies.
2
Other Conditioning Programs – Actimab-MDS and Iomab-ACT for Gene Therapy
We have completed our interactions with FDA and have a clear pathway to a pivotal trial after a short dose confirming Phase 1 study (which we believe will likely be 4uCi/kg body weight) for the Actimab-MDS targeted conditioning study in high-risk patients with MDS or myelodysplastic syndrome. The start of the gene therapy trial in HIV related lymphoma has been delayed as the University of California Davis was severely impacted due to COVID-19 and cohort expansion in the trial which uses chemotherapy conditioning, highlighting the need improved conditioning regimens. We will update on these programs as they move ahead in 2021.
CD33 Program Revitalization Via Therapeutic Combination Trials Data
Our CD33 program is showing compelling promise and could become an important driver of value. Recall, through the Phase 2 trial, we demonstrated that single-agent Actimab-A was extremely potent (ORR of 69%) and had no extramedullary toxicity outside of myelosuppression. We are parlaying this profile via a therapeutic combination strategy with established agents in R/R AML. As very few patients are cured with the 9 approved drugs, R/R AML patients account for almost 70% of the AML population. Each of our Actimab-A combination trials adds low, sometimes sub-therapeutic doses of Actimab-A, to established doses of drugs or drug cocktails and delivers internalized radiation to a highly radiation sensitive liquid cancer. There is also a mechanistic synergy or potentiating effect possible from adding Actimab-A in the two combination trials with CLAG-M and venetoclax. Actimab-A, in addition to its single agent activity can damage certain proteins like Mcl-1 and act in concert with DNA damage response inhibitor drugs and yield extremely high patient response rates without compromising their safety.
Supporting our approach are data from the Actimab-A + CLAG-M trial which showed a very high remission rate of 86%, 60% higher than CLAG-M alone. This second dose cohort combined a sub-therapeutic dose of Actimab-A with a standard CLAG-M regimen. Further, the minimal residual disease or MRD negative rate was an impressive 71%, which bodes well for durability of response. Venetoclax combinations are arguably the most widely prescribed regimen but most patients ultimately fail, and our trial is attracting significant investigator interest which has led to site expansion. We are excited as we believe these data events can demonstrate the immense value creating potential of our CD33 program, which has been largely overshadowed by Iomab-B and is deserving of further attention. We look forward to presenting updated POC data from our CLAG-M combination and first human data from the venetoclax combination Phase 1 trial by year-end.
Why Bolster R&D Capabilities at Actinium?
Actinium the company, was formed two decades ago to harness the power of radioisotopes, in particular Actinium-225 or Ac-225, to treat and potentially even cure cancer. The idea was far ahead of its time and the company certainly had its share of vicissitudes…we are the sixth management team. We restarted R&D three years ago, within a few months of our tenure, and have revitalized the aging patent portfolio, informed CD45 and CD33 program expansion via our research and entered into a research partnership with a global biopharma company. We now execute in a highly collaborative manner navigating complex scientific considerations, informed by commercial sensitivities, our clinical know-how and supply chain expertise.
3
While no one has cured cancer, not even with a radiotherapeutic since Actinium was formed, there have been certain high-profile successes with radiopharmaceuticals that have created tremendous value for shareholders. This success has attracted new entrants and significant investments. Given a fortified balance sheet and potential for significant value creating clinical milestones by year-end that could enable us to build a specialty oncology company, we have begun stage 2 of our R&D plan. We have elected to develop internal laboratory capabilities to be able to execute our plans more efficiently than using outside vendors. We believe that this internal R&D capability is essential to attract collaborators, accelerate planned clinical programs and strengthen our competitive position as a leader in developing Ac-225 based therapeutics.
We are proud of the progress we have made in 2020 in the face of tough operating conditions and are excited for year-end clinical milestones that can set the stage for a potentially transformative 2021. Our strong balance sheet, enhanced team and capabilities, position your company well for value creation.
On behalf of our entire company and the Board of Directors, I thank you for your commitment to Actinium Pharmaceuticals and supporting our mission to develop medicines in areas where our product candidates can materially improve patient lives.
Sincerely,
/s/ Sandesh Seth
Sandesh Seth
Chairman and Chief Executive Officer
| Key Achievements in 2020 | Key Upcoming Milestones | |||
 |
Iomab-B mid-point analysis of SIERRA | ●Iomab-B SIERRA 75% patient data | 4Q:2020 | |
 |
Exercised ad hoc analysis for SIERRA | ●Iomab-B SIERRA Ad Hoc Analysis | 4Q:2020 | |
 |
Raised ~$60M in 1H:2020 | ●Actimab-A CLAG-M POC | 4Q:2020 | |
 |
Completed Phase 1 Actimab-A CLAG-M | ●Actimab-A Venetoclax 1st human data | 4Q:2020 | |
 |
Initiated Actimab-A Venetoclax combo | ●Actimab-A Venetoclax POC | 2021 | |
 |
Launched research facility | ●Iomab-ACT CD19 CAR-T POC | 2021 | |
 |
Iomab-ACT CAR-T Collaboration | ●Actimab-A Gene Therapy update | 2021 | |
4