
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
| ☐ | Preliminary Proxy Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ☒ | Definitive Proxy Statement |
| ☐ | Definitive Additional Materials |
| ☐ | Soliciting Material under Rule 14a-12 |
Actinium Pharmaceuticals, Inc.
(Name of Registrant as Specified In Its Charter)
N/A
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ | No fee required. | |
| ☐ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |
| 1. | Title of each class of securities to which transaction applies: | |
| 2. | Aggregate number of securities to which transaction applies: | |
| 3. | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): | |
| 4. | Proposed maximum aggregate value of transaction: | |
| 5. | Total fee paid: | |
| ☐ | Fee paid previously with preliminary materials. | |
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-1 1(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |
| 1. | Amount Previously Paid: | |
| 2. | Form, Schedule or Registration Statement No.: | |
| 3. | Filing Party: | |
| 4. | Date Filed: | |
November 16, 2018

Dear Fellow Shareholders:
It has been a critical year for Actinium as your management team have developed a new strategy and focus for your company. This refocused strategy has been driven in large part by a strengthened leadership team, valuable clinical data and a good deal of foresight.
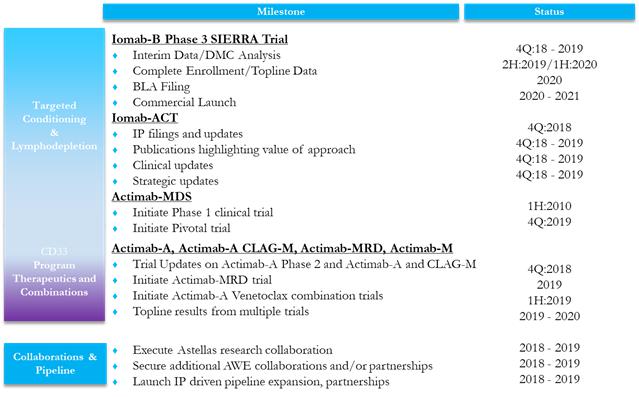
Today, Actinium’s pipeline is refocused on two key areas: first, targeted conditioning prior to adoptive cell therapies such as BMT or bone marrow transplant and CAR-T; and second, on therapeutic combinations with our ARCs or Antibody Radiation Conjugates. We are optimistic that executing toward key milestones across a multi-asset, multi-disease pipeline will provide clinically positive outcomes and yield positive returns for shareholders. We encourage you to read the list of Actinium’s expected milestones in 2019 in Appendix A that have been made possible by our many accomplishments in 2018 which we have highlighted in Appendix B.
We recognize that the SIERRA trial has taken longer than originally expected and understand how this could create angst for our shareholders. We share your frustration and we thank you for your continued support and greatly appreciate your patience. Without appearing sanguine, we believe that the SIERRA trial is on solid footing under a stronger team after we have restructured and rebuilt various aspects of the company, particularly the clinical organization. This has occurred while simultaneously forging ahead on multiple fronts across our pipeline, which has resulted in a much stronger strategic focus for the company in 2018. Let us also take comfort in the heartening interim feasibility and safety data from the Iomab-B SIERRA trial that has been accepted for oral presentation at the American Society of Hematology (ASH) Annual Meeting in early December. Today, we feel confident that we have the right team in place and that the changes we have made were necessary to deliver the positive data we look forward to presenting. Overall, we are proud of our accomplishments in 2018 and are energized by our refocused vision and the opportunities that have been created.
In 2018 we were able to build the leading franchise in targeted conditioning. With our Phase 3 Iomab-B as the foundation, we added two new programs: Iomab-ACT for lymphodepletion prior to CAR-T and near-pivotal Actimab-MDS for conditioning high-risk patients with myelodysplastic syndrome prior to a BMT. These additions afford us a multi-asset, multi-disease pipeline that is unrivalled in the industry. Each of these programs offers the potential to improve patient access and outcomes based on the superior ability of our ARCs to safely and efficiently deliver the appropriate dose of radiation needed to condition the marrow or lymph system compared to chemotherapy. Chemotherapy, which currently is used as the standard of care, has side effects as a result of being non-targeted. These side effects limit both outcomes and eligibility of patients, especially older adults. What is most exciting is that these new programs not only hold the potential to treat a significant unmet medical need in a broad population, but they provide Actinium with the potential for multiple product launches and label expansion initiatives in the two to three years starting in 2020.
At ASH, we will demonstrate that Iomab-B feasibility and safety interim results look very promising during our high-visibility, oral presentation for our Phase 3 SIERRA trial. There are multiple interim milestones for safety and efficacy for Iomab-B through next year, including trial completion. In addition, we are preparing for Actimab-MDS to enter a pivotal trial after a short dose-confirmatory Phase 1 trial planned in 2019. We also have several Iomab-ACT clinical events planned as outlined in our key milestones section in Appendix B. These value creating events also will afford us important opportunities to engage in discussions with various constituencies who can support our goals, including potential collaborators. We believe that the highly differentiated focus of our pipeline, the lack of visible competition, and the high-value potential of our products presents a very real opportunity for Actinium to play a leadership role in addressing this area of high unmet medical need.
The therapeutic combinations approach affords us yet another opportunity to use the potential of our ARCs in combination with other chemotherapeutic or immuno-oncology-based drugs. Examples are the trials we are doing with Actimab-A and the CLAG-M regimen and with the high-profile drug Venclexta or venetoclax. Combination approaches to oncology drug development have are increasingly common, especially with larger companies in the immuno-oncology field. Radiation therapy is used effectively in solid tumors, but it is not a viable option for blood cancers, which are too diffuse to be treated with external radiation that cannot be targeted. Our ARCs have the ability to target the radiation to be delivered in a safe manner. The possibility of adding a highly-effective, potentially synergistic modality such as radiation to immuno-oncology drugs via an ARC, as we have demonstrated pre-clinically with Actimab-A and venetoclax, has the potential to lead to superior combinations and superior outcomes. We look forward to the several data events from our combination trials and other CD33 program expansion trials, and to making our technology and CD33 program available to collaborators as part of our value-creating strategy.
Our new strategy has been enabled in part due to our Antibody Warhead Enabling, or AWE, platform in combination with a revitalized research capability that we built this year at Actinium. As a result, we were able to enter into a collaboration with the Top-20 big-pharma company Astellas. Further, in a few short months, we were able to conduct validating experiments that supported the Iomab-ACT program for CAR-T and combination trials with venetoclax, file the patents to protect these ideas, and make these programs known publicly. As a result of these and other research activities, we will continue to strengthen our leading AWE technology platform and we expect it to be a profit center for the company along with the Iomab-ACT program. Recent strategic activity in the radiopharmaceutical space is contributing to growing recognition and acceptance of the value of targeted radiation among large and medium-sized biopharma companies. Actinium is well positioned to address these needs “in the age of Radiopharma 2.0”. (Please see Appendix C for a list of FAQs that we think put in perspective the rapid pace of Actinium’s accomplishments and the positive impact we anticipate these changes will support).
Certainly, it was no small feat to develop a new strategic focus, launch new clinical trials, advance existing trials toward clinical milestones while simultaneously recruiting for and restructuring our small, 30-person team. Today, we have successfully reinvigorated Actinium’s research team. We also have made advances in securing our intellectual property and have entered into an important and validating collaboration. We are proud that a 30-person company, with a just-in-time, personalized medicine supply chain, can support not only an ongoing Phase 3 trial that is showing great promise, but also several phase 1/proof of concept trials, all boding well for truly transformational results.
Due to our efforts this year, Actinium is well-positioned for an exciting future built on strong science, positive clinical data, and a committed and talented team. We want to reiterate that besides our differentiated focus on targeted conditioning with a multi-asset, late-stage pipeline with visible and promising clinical data, our technological leadership extends to expertise in alpha-radiation with the work we are doing in our Ac-225 isotope program. The scarcity value of independent companies, which is the result of a number of acquisitions in the space, also contributes to a much more attractive profile for our company. These factors are only just becoming apparent to investors and strategic players as we have only recently completed the refocusing and have now begun an extensive and months-long process of educating people about the “new Actinium.” Taking this into account, we are requesting your support for all of our proposals in the proxy card, some of which are being requested to enable the company to be in the best position to protect shareholder interests in the event certain strategic actions occur.
We are pleased to be able to report that progress has been made to set up the pipeline for success with multiple value-generating drivers before year-end, into 2019 and beyond. We thank you for your continued support and belief in our drug candidates, technologies and efforts, and hope you are as excited and optimistic as the team at Actinium is about the year ahead and the longer-term future of your company.
On behalf of Team Actinium.
Respectfully,
Sandesh Seth
Chairman and Chief Executive Officer

Appendix A – Key Milestones for 2019

Appendix B – Key Achievements in 2018
Significantly strengthened our leadership team and capabilities bringing decades of experience to Actinium resulting in a new level of execution across the Company
| - | Enhanced Transplant Expertise to Support Strategic Refocus: Added significant bone marrow transplant expertise to our clinical development team to support our strategic focus in targeted conditioning. This has allowed us to expand and develop a franchise opportunity with three targeted conditioning programs including Iomab-ACT for CAR-T that progressed from conceptualization to launch in less than a year, near-pivotal Actimab-MDS for conditioning high-risk patients with myelodysplastic syndrome prior to a BMT and Phase 3 Iomab-B as the foundation. | |
| - | Strengthened Clinical Operations to Strengthen Trial Execution and Pipeline Expansion: We have made key hires including a head of clinical operations as a new function within Actinium. The strengthened team is dedicated to the efficient, timely and cost-effective execution of our clinical trials and in a short time has developed and is implementing initiatives designed to complete enrollment of the SIERRA trial as quickly as possible. | |
| - | Established Research Team: Reinvigorated our research efforts by establishing a research group. This enabled our work with Ac-225 labeled daratumumab and led to our first publication at AACR and new patent filings that extend our IP portfolio in line with our strategic vision. Our research team is working in alignment with our clinical development team resulting in highly supportive data for our Iomab-ACT program and the Actimab-A plus Venetoclax combination trials. The improved internal alignment also benefits our business development efforts. |
Executed our first AWE platform partnership with Astellas Pharma, Inc., a Top 20 global biopharma company
| - | Validates the utility of our technology platform and its potential value to large biopharma companies as well as our leadership position with the Ac-225 isotope. |
Began a combination trial with Actimab-A and CLAG-M
| - | This trial studies our ARC approach with other modalities, in this case chemotherapy, where we expect to see synergies that can improve patient outcomes. |
Launched the Actimab-A MRD trial for a significant unmet need and potentially large market opportunity
| - | Aligns our clinical development with key advancements in the field as minimal residual disease or MRD is becoming an increasingly important biomarker and emerging endpoint. Success of this trial implies a large market opportunity as frequent dosing of Actimab-A could be required to maintain a disease free or MRD negative AML state. |

Initiated our Iomab-ACT program for targeted lymphodepletion for CAR-T therapies
| - | Builds on our targeted conditioning strategy by leveraging our clinical experience with Iomab-B to position us as a potentially universal solution for targeted lymphodepletion with improved access and outcomes. Actinium is at the forefront with this solution for the large and rapidly growing CAR-T industry. |
Successfully completed the Actimab-A Phase 2 trial as a single agent in a difficult-to-treat patient population with identification of an attractive future development pathway
| - | Strong single agent activity and minimal extramedullary toxicities in the Phase 2 Actimab-A AML trial paved the way for key opinion leaders to support our Actimab-MDS trial for targeted conditioning and our two Actimab-A plus Venetoclax combination trials. This pathway forward differentiates the asset and provides an attractive and high-value route for further development compared to a high-risk, controlled Phase 3 trial for AML patients. |
Announced two clinical trials that will study Actimab-A with Venetoclax, a targeted therapy
| - | Further aligns Actinium with the most recent advancements in the AML field and with the support of thought leading physicians from MD Anderson Cancer Center and UCLA Medical Center. |
Positive outcomes from our interactions with the FDA regarding Actimab-MDS
| - | Resulted in an accelerated regulatory pathway that will now consist of a small dose finding Phase 1 trial before moving to a pivotal trial for our second targeted conditioning indication. Also resulted in a broader patient population than what was proposed to FDA. |
Multiple abstracts accepted at for ASH including the acceptance of preliminary feasibility and safety results of the Iomab-B Phase 3 SIERRA trial for oral presentation
| - | Gives Iomab-B and the ongoing SIERRA trial significant exposure at ASH, the largest blood cancer-focused medical conference in the world, where only approximately 10% of accepted abstracts are elevated to oral presentations. |

Appendix C – FAQ’s or Frequently Asked Questions
How has Actinium transformed itself from a year ago? What is the new focus?
November 2017 Pipeline
At this time last year, we had 3 trials in our pipeline, 1 targeted conditioning trial and 2 single-agent therapeutic trials.
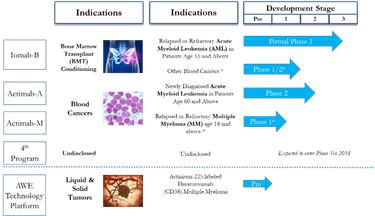 |
♦ Iomab-B was our only targeted conditioning program with the Phase 3 trial in the early stages of enrollment ♦ Our CD33 program had just been expanded to Multiple Myeloma ♦ Early efforts with our AWE platform had been initiated
|
November 2018 Pipeline
Fast forward to today and we are advancing 3 targeted conditioning trials (1 pivotal and 1 near-pivotal) 3 therapeutic combination trials and 2 single-agent therapeutic trials in highly differentiated indications.
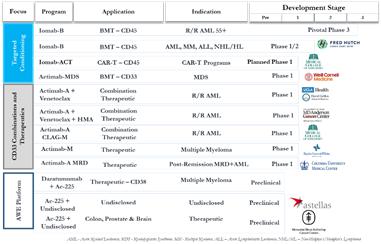 |
♦ Targeted conditioning program expanded to 3 trials including Iomab-ACT for CAR-T and near pivotal Actimab-MDS ♦ SIERRA trial reached 25% enrollment with interim data to be presented in oral presentation at ASH ♦ CD33 program expanded to best in class with the largest addressable patient population and breadth of applications ♦ AWE platform validation via our collaboration with Astellas and expanded research efforts
|

We have expanded our pipeline efficiently and cost-effectively by leveraging the strengths of our ARC candidates without undertaking significant de novo development by utilizing our AWE platform and enhanced R&D capabilities to support these new initiatives. Because our CD45 and CD33 targets are applicable to multiple diseases and indications, each of our ARCs become a pipeline within a drug. We believe there is much more still to come. Given our ARC approach, we can utilize a high-dose to facilitate targeted conditioning and low-dose strategy to leverage the proven modality of radiation for novel therapeutic combinations.
Targeted Conditioning Related FAQs
Why Is Targeted Conditioning so attractive?
We focus on targeted conditioning because it enables treatments that are potentially curative in nature, such as BMT and CAR-T, for a significant number of patients with a range of diseases. In advance of BMT or CAR-T, patients must have their bone marrow and immune system conditioned to make room for the new cells. This is done today with non-targeted chemotherapy and external beam radiation. Our targeted conditioning approach delivers potent radiation to specific cells to enable more effective conditioning while at the same time minimizing effects to normal cells with the hopes of having the strongest and healthiest patient prior to their BMT or CAR-T. Our focus on targeted conditioning sets us apart as we believe we are the only company with a multi-disease, multi-target, late-stage pipeline for targeted conditioning. Further, we are not aware of any other company with a focus on targeted conditioning that is as advanced as we are in clinical trials. Our antigen targets CD45 and CD33 are widely expressed in many hematologic indications. We believe there is an opportunity to expand the addressable patient population for our programs. For example, CD45 is expressed on leukemia cells, lymphoma cells and multiple myeloma cells and significant data has been generated in these indications at the Fred Hutchinson Cancer Research Center which we can use for label expansion of Iomab-B. With CD33 we are targeting high-risk MDS patients with the Actimab-MDS program. Because the addressable market for our targeted conditioning drug candidates is limited to 50-100 medical centers that perform a majority of BMT and CAR-T procedures, we believe we have a tremendous opportunity to create a leading, independent organization in this space that has little competition.
What was accomplished via the Iomab-B and the SIERRA Trial? What can we expect in future?
We have strong talent supporting the SIERRA trial. Our team includes a transplant physician who has more than 20 years of clinical experience and a Head of Clinical Operations, a new position, who brings to Actinium more than 25 years of experience. Both of these clinical experts are focused on the trial’s execution and completion. In addition, a third M.D. and a nurse educator who has significant oncology drug experience are focused on providing training and support to trial sites. Finally, we have two clinical research associates dedicated to the operations of the SIERRA trial. Effectively, the clinical team was restructured in April of this year and the average tenure for the team members is seven months. In this short time, this new team has positively impacted execution of the SIERRA trial and we have great confidence in their capabilities.

We expect to achieve the following milestones going forward as indicated in Appendix B and below. We look forward to updating you as each of these milestones is reached and believe the analyzed data at each point will provide many valuable insights into this important trial.
| ● | Enrollment of the 70th patient – an efficacy and safety analysis may occur when the 70th patient reaches the primary endpoint | |
| ● | 50% Enrollment – a DMC safety analysis will occur just like the analysis that occurred after 25% of patients were enrolled | |
| ● | Enrollment of the 110th patient – a second efficacy and safety analysis may occur when the 110th patient reaches the primary endpoint | |
| ● | 75% Enrollment – the third and final safety analysis | |
| ● | Completion of Enrollment – a major milestone in any trial but perhaps more so with SIERRA as it is the only trial focused on targeted conditioning for older patients with active, relapsed or refractory AML. |
What is the value of the Iomab-ACT program?
We believe our Iomab-ACT program has the potential to offer a chemotherapy-free conditioning regimen prior to CAR-T that can effectively achieve lymphodepletion in a single-dose and in an outpatient setting. Current approaches for lymphodepletion rely on chemotherapy, typically the combination of fludarabine and cyclophosphamide or Fly/Cy, which is non-specific, toxic and sub-optimal. We believe that lymphodepletion with the Iomab-ACT construct will result in superior outcomes from CAR-T including improved patient responses and long-term outcomes, increased access to CAR-T and reduced toxicities associated with CAR-T. We believe the Iomab-ACT program can be a universal solution for all CAR-T therapies and a potentially valuable expansion of our targeted conditioning franchise. In addition, the Iomab-ACT program represents the new level of innovation and execution that exists within Actinium as this concept went from ideation to existence in rapid fashion by leveraging the deep clinical experience and data of our CD45 program and the expanded research capabilities of the company. As we have done with all of our latest initiatives, we have generated an intellectual property portfolio which in this case currently encompasses six patents pertaining to the Iomab-ACT program. We are committed to improving patient outcomes and generating value from our Iomab-ACT program in a rapid fashion with this being a top priority for Actinium in 2019.
What is Actimab-MDS expected to add?
We believe it is rare for a 30-person biotech company to have the opportunity to have not just one but two pivotal trials in a field by itself. This is the opportunity created by Actinium in 2018 by adding Actimab-MDS to our clinical pipeline. Just as we did with the Iomab-ACT program where we leveraged our experience with CD45 and Iomab-B, with Actimab-MDS, we leveraged the extensive experience with CD33, the isotope Ac-225 and Actimab-A to develop this exciting opportunity.

The vision that brought the Actimab-MDS into a clinical trial was the result of our team and our collaborators’ ability to take the seeming limitation of Actimab-A - namely prolonged myelosuppression - and consider the possibility that it could be a useful attribute in a setting in which myelosuppression is not a limitation but a desired outcome. This was in the context of using highly myelosuppressive doses of the construct to myeloablate or “burn out” the bone marrow prior to a bone marrow transplant. A great deal of credit is due to Dr. Gail Roboz, Director of Leukemia at Weill-Cornell Medical Center, who conceptualized the idea after serving as an investigator in our Actimab-A Phase 2 trial. This trial enrolled patients whose MDS had progressed to AML. As we have reported, Actimab-A had potent myelosuppression capabilities with minimal toxicities outside of the bone marrow. Recognizing that a bone marrow transplant is the only potentially curative treatment option for patients with high-risk MDS and that myelosuppression can be alleviated with a bone marrow transplant, the decision to advance this trial was made.
Having been guided toward a significantly faster regulatory pathway by the FDA than we had originally expected, we plan to conduct a pivotal trial after completing a small Phase 1 trial to confirm the myeloablative dose. Originally, we had proposed a 60-80 patient Phase 2 trial that would then be followed by a pivotal trial. Also, we had originally proposed a patient population limited to only those patients with a specific mutation to the TP53 gene, but the FDA guided that we should expand the addressable patient population to intermediate and high-risk MDS patients. In 2019, we look forward to working with Dr. Roboz and her colleagues from the MDS Clinical Research Consortium to complete the Phase 1 trial, with the goal of moving toward Actinium’s second pivotal trial.
Actimab-A AML Phase 2 Trial and CD33 Program Questions
What did the Actimab-A Phase 2 trial in AML yield? What happens next?
We are excited that the Actimab-A Phase 2 AML trial demonstrated the potent single-agent activity of the Ac-225 – lintuzumab targeting agent. However, in the older unfit patient population enrolled in the Phase 2 trial, myelosuppression proved challenging. Rather than move ahead into a large, lengthy and expensive Phase 3 trial in the increasingly crowded field of AML therapies, we made the decision to follow the signals the drug candidate was giving us and move into what we believe to be more attractive opportunities with this agent at different doses in targeted conditioning and in combination with other drugs. This decision led to our Actimab-MDS trial giving us our second targeted conditioning asset. Actimab-MDS will utilize a high dose of the Ac-225 – lintuzumab construct to achieve effective myeloablation for high-risk MDS patients prior to a bone marrow transplant.

At a lower dose of the construct, we see the opportunity to use Actimab-A in combination with chemotherapies, targeted therapies and immunotherapies. We have already begun executing on this strategy with our three exciting combination trials. As a result, we have aligned Actinium with targeted therapies that will allow us to generate additional data that could prove valuable to potential partners. We have seen increased activity in targeted radiation both in terms of company acquisitions and research publication volume that we believe is creating a ground swell of interest for this approach amongst the industry. We believe we are solidly positioned at this opportune time given the breadth of our pipeline, versatility of our platform and strong IP portfolio.
What is the attractiveness of combinations? Why does the industry care?
Therapies based on the combination of two or more agents have long been used in the treatment of patients with cancer with the goal that a synergistic effect would emerge and the use of combination therapies continues to grow rapidly. Our ARC drug candidates utilize the power of radiation, which is a proven therapeutic modality that is used to treat more than half of all cancer patients. However, we harness the powers of radiation inside the body in a targeted manner, thereby eliminating the side effects that come with delivering radiation to cancer cells from outside the body. It has been demonstrated that radiation causes cancer cell DNA and a tumor’s microenvironment to work in synergy with other agents. We believe better outcomes are possible through Ac-225 – Lintuzumab in certain cancers cells that express the CD33 antigen because it delivers radiation in a way no other drug candidate can to these cancer cells that are radiation sensitive. This is the basis for our combination trials with Venetoclax and CLAG-M, both of which are grounded in strong scientific rationales as well as preclinical and clinical data.
We feel that demonstrating the synergy of our ARCs with other therapeutic modalities will increase their attractiveness to potential partners. For instance, if we could demonstrate that Ac-225 can make cancer cells and or tumors “hot,” that is to say more noticeable to the immune system, we believe that would be of interest to the large universe of big pharma companies that have strong immuno-oncology franchises.
How do you support your claim that you have a CD33 Program that is best in class? What can we expect from this program?
The CD33 field is largely dominated by big pharma and biotech companies that are partnered with larger biopharma firms. These companies are focused on AML and employ antibody drug conjugate, bispecific antibody and naked antibody approaches. Actinium is the only company utilizing an antibody radiation conjugate.

CD33 AML Programs
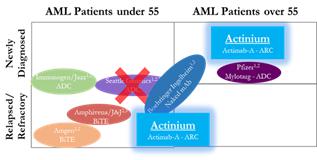
Through our ARC approach we have expanded our CD33 program from a single trial in AML to now six trials that are ongoing or planned for 2019. Our ARC technology allows us to move into indications that other CD33 program sponsors have not been able to address because of the inherent limitations of their technological approaches. We believe our CD33 program is best in class because it not only addresses three diseases; AML, MDS and Multiple Myeloma, which is the broadest in scope compared to competing CD33 focused programs and provides for the largest addressable patient population or market opportunity. Further, it is the only CD33 program for patients with Multiple Myeloma and the only CD33 program for targeted conditioning.
Actinium’s Multi-Disease, Multi-Indication CD33 Program
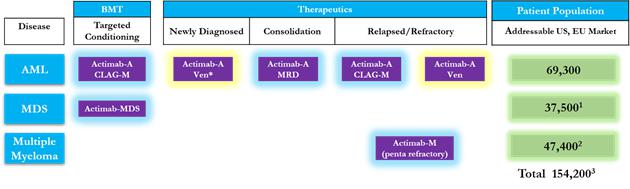
Given our extensive experience and clinical data from our CD33 program, we believe this can be leveraged to move into new indications and start new trials in a cost-effective manner without having to fund de novo development. We will continue to identify applications and indications for our CD33 program that build on its best-in-class profile. Given that our CD33 program is the most advanced and has the broadest scope, companies seeking to enter this area may find our program very attractive. Further, our focus on therapeutic combinations allows us to engage with potential partners who are interested in the radiation modality for combinations with their therapeutic modalities, which we believe can leveraged strategically to drive value.
How does the AWE Platform add value?
Our AWE platform is an engine that can drive growth in our pipeline as well as collaborations and partnerships. We view the AWE platform as an immensely valuable asset that is now being leveraged properly under the stewardship of our dedicated, talented and experienced research team. Our AWE platform underpins our clinical programs and is supported by extensive preclinical data, clinical data and 75 patents. We will continue to build our intellectual property portfolio to further bolster AWE’s profile as we seek to monetize it.
We believe AWE can drive partnerships in numerous ways including our biobetter strategy. This strategy takes an established biologic drug and using a radioisotope like Ac-255, we demonstrate enhanced potency, efficacy or improved administration. We demonstrated this with our work labeling daratumumab, the blockbuster CD38 antibody therapy for multiple myeloma that is marketed as Darzalex by Johnson & Johnson. In this case, we were able to increase cell killing and demonstrate efficacy on cell lines that displayed resistance to unlabeled daratumumab.
Another approach is to find antibodies or other targeting agents that are no longer being pursued by their pharma or biotech sponsor and reinvigorate them given that ARC’s cell killing capabilities, which are not dependent on genetic factors, high antigen density, and do not require internalization of the target. These attributes are major differentiators from other modalities like antibody drug conjugates or ADCs. Finally, we can work with collaborators to research our radioisotope-based warhead payloads in conjunction with novel targeting agents. We have already established a track record in this regard through our research collaboration with Astellas that resulted in non-dilutive capital from Astellas and ongoing funding.
Why do you say the Astellas transaction was “particularly validating”?
The decision by Astellas to collaborate with Actinium is the first instance of corporate validation of our alpha platform technology. We are very pleased and honored to work with a leading innovator and science-driven company like Astellas. Astellas had previously prioritized ADC or Antibody Drug Conjugate technology with its purchase of Agensys Inc. for over $400MM. Subsequently, Astellas appears to have disinvested in this approach and has announced a wind-down of this effort. We believe that the decision to pursue an ARC development approach by a knowledgeable company like Astellas who certainly has considerable experience with alternative ADC technology is a tacit acknowledgement of the inherent advantages of ARC technology as a targeting agent. Further, the selection of Actinium by such an experienced player is a testimony to our AWE platform for sure but also our research capabilities and due to our not being a part of a larger company. Other factors that were considerations in selection of Actinium that will be relevant for future AWE partnerships are the clinical validation of the safety and efficacy of our linker technology and our years of experience in handling the isotope Ac-225 and the patent portfolio surrounding it. Further, we believe that our stage-appropriate supply chain, which has the demonstrated capability of manufacturing and supplying radiolabeled drug to top cancer centers across the U.S. is a major point of differentiation for Actinium and will be a consideration for most partners unless they wish to invest years and millions of dollars to acquire such a capability.

How is the R back in R&D at Actinium? What can we expect going forward?
Prior to the last 12 months, Actinium’s efforts focused largely on clinical development of existing trials. In 2018, with our renewed focus on research we have generated new IP, demonstrated the utility of our AWE platform, signed our first collaboration with a top 20 pharma company, supported the launch of Iomab-ACT and supported our Actimab-A and Venetoclax combination trials.
We believe that our platform has immense potential for liquid and solid tumors with the flexibility to attenuate our dose for desired outcomes like our high-dose myeloablation/low-dose lymphodepletion strategy with Iomab. We will continue to explore new indications for our existing focus on CD45 and CD33 and CD38 targets, file new IP and work towards additional collaborations and partnerships.
We have heard the phrase “it is the age of Radiopharma 2.0 now” being used recently? Please explain and what does it mean for Actinium?
In the last 12 months alone, we have seen two multi-billion-dollar acquisitions of companies with radiopharmaceutical-based therapies. The first being Advanced Accelerator Applications, Inc., acquired by Novartis for $3.9 billion and the most recent being Endocyte, also acquired by Novartis, for $2.1 billion. These follow Algeta that was acquired by Bayer in 2013 for $2.9 billion. There are also a growing number of publications demonstrating the utility of radiotherapy in combination with other modalities which is creating further interest in this technology.
As a result of these acquisitions, there are just a few unpartnered radiopharmaceutical therapies remaining, creating a scarcity of assets in the field. From our assessment of the landscape, we believe we have the broadest, most late-stage pipeline which addresses a large patient population with unmet or underserved needs. Iomab-B, Actimab-MDS and the Iomab-ACT program provide Actinium the only multi-disease, multi-target pipeline for targeted conditioning that is intended to improve access and outcomes to potentially curative cellular therapies such as bone marrow transplant and CAR-T.
We believe that our targeted conditioning pipeline allows Actinium a viable pathway to commercialize these drug candidates as an independent company and without a partner. However, we also recognize that at the right time our pipeline may be recognized as a strategic business unit opportunity for a strategic partner. Further, our best in class CD33 therapeutics program which will also have multiple data readouts in the 2019-2020 timeframe may further increase attractiveness as a partner for larger companies seeking a revenue base with differentiated assets which we have in plenty along with our AWE platform which can generate further opportunities.

TABLE OF CONTENTS
| Page | |
| General | 1 |
| Questions and Answers | 1 |
| Who Can Help Answer Your Questions? | 5 |
| Corporate Governance | 5 |
| Board Committees | 7 |
| Director Compensation | 9 |
| Audit Committee Report | 9 |
| Compensation Committee Report | 11 |
| Directors and Executive Officers | 15 |
| Executive Compensation | 20 |
| Principal Stockholders | 27 |
| Certain Relationships and Related Transactions | 29 |
| Proposal 1 — Election of Directors | 29 |
| Proposal 2 — Ratification of the Appointment of Marcum LLP | 31 |
| Proposal 3: To approve an amendment to the Actinium Pharmaceuticals, Inc. 2013 Amended and Restated Stock Plan, as amended, to increase the shares of our common stock available for issuance thereunder by 5 million shares to support planned hiring efforts as the company grows | 32 |
| Proposal 4: To approve an amendment to our Certificate of Incorporation to increase the number of shares of common stock, par value $0.001 per share, the corporation is authorized to issue by 200,000,000 shares | 36 |
| Other Matters | 38 |
| Annual Report on Form 10-K | 38 |
| Householding of Proxy Materials | 38 |
| Electronic Delivery of Company Stockholder Communications | 38 |
| Important Notice Regarding the Availability of Proxy Materials for the Stockholders Meeting to be held on December 18, 2018 | 39 |
| Proposals of Stockholders | 39 |
| Where You Can Find More Information | 39 |
Stockholders Should Read the Entire Proxy Statement Carefully Prior to Returning Their Proxies
i
PROXY STATEMENT
FOR
ANNUAL MEETING OF STOCKHOLDERS
GENERAL
The enclosed proxy is solicited on behalf of the Board of Directors of Actinium Pharmaceuticals, Inc. for use at our annual meeting of stockholders to be held at The Garden City Hotel, 45 Seventh St, Garden City, NY 11530 on December 18, 2018 at 9:30 a.m. Eastern Standard Time. Voting materials, including this proxy statement and proxy card, are expected to be first delivered to all or our stockholders on or about November 8, 2018.
QUESTIONS AND ANSWERS
Following are some commonly asked questions raised by our stockholders and answers to each of those questions.
What may I vote on at the annual meeting?
At the annual meeting, stockholders will consider and vote upon the following matters:
Proposal 1: To elect Sandesh Seth and Jeffrey W. Chell, M.D. as Class II directors to serve for a three-year term that expires at the 2021 Annual Meeting of Stockholders, or until his successor is elected and qualified or until his earlier resignation or removal; and
Proposal 2: To ratify the appointment of Marcum LLP as our independent registered public accounting firm; and
Proposal 3: To approve an amendment to our Actinium Pharmaceuticals, Inc. 2013 Amended and Restated Stock Plan, as amended, to increase the shares of our common stock available for issuance thereunder by 5 million shares, to support planned hiring efforts as our company grows; and
Proposal 4: To approve an amendment to our Certificate of Incorporation to increase the number of common stock, par value $0.001 per share, we are authorized to issue by 200,000,000 shares; and
To consider and act upon any other business as may properly come before the annual meeting or any adjournments thereof.
How does the Board of Directors recommend that I vote on the proposals?
Our Board unanimously recommends that the stockholders vote “FOR” all proposals being put before our stockholders at the Meeting.
How do I vote?
Whether you plan to attend the annual meeting or not, we urge you to vote by proxy. If you vote by proxy, the individuals named on the proxy card applicable to your class of stock, or your “proxies,” will vote your shares in the manner you indicate. You may specify whether your shares: should be voted for or withheld for the nominee for director; should be voted for, against or abstained with respect to the ratification of the appointment of the Company’s independent registered public accounts; should be voted for, against or abstained with respect to approving an amendment to our Actinium Pharmaceuticals, Inc. 2013 Amended and Restated Stock Plan, as amended; and should be voted for, against or abstained with respect to approving an amendment to our Certificate of Incorporation to increase the number of common shares we are authorized to issue to 600,000,000 shares. Voting by proxy will not affect your right to attend the annual meeting. If your shares are registered directly in your name through our transfer agent, Action Stock Transfer Corporation, or you have stock certificates registered in your name, you may submit a proxy to vote:
By Internet or by telephone. Follow the instructions attached to the proxy card to submit a proxy to vote by Internet or telephone.
| 1 |
By mail. If you received one or more proxy cards by mail, you can vote by mail by completing, signing, dating and returning the enclosed proxy card applicable to your class of stock in the enclosed postage prepaid envelope. Your proxy will be voted in accordance with your instructions. If you sign the proxy card but do not specify how you want your shares voted, they will be voted as recommended by our Board of Directors.
In person at the meeting. If you attend the annual meeting, you may deliver your completed proxy card in person or you may vote by completing a ballot, which will be available at the annual meeting. You are required to register in advance of the annual meeting if you plan to attend the annual meeting in person. If you wish to register in advance of the annual meeting, please contact our investor relations office by no later than December 11, 2018, by e-mail to soloughlin@actiniumpharma.com, mail to Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York, New York 10016, or telephone at (646) 677-3875.
Telephone and Internet voting facilities for all stockholders of record will be available 24-hours a day and will close at 11:59 p.m., Eastern Standard Time, on December 17, 2018.
If your shares are held in “street name” (held in the name of a bank, broker or other nominee who is the holder of record), you must provide the bank, broker or other nominee with instructions on how to vote your shares and can do so as follows:
By Internet or by telephone. Follow the instructions you receive from the record holder to vote by Internet or telephone.
By mail. You should receive instructions from the record holder explaining how to vote your shares.
In person at the meeting. Contact the broker, bank or other nominee who holds your shares to obtain a broker’s proxy card and bring it with you to the annual meeting. You will not be able to vote at the annual meeting unless you have a proxy card from your broker, bank or other nominee.
What happens if additional matters are presented at the annual meeting?
Other than the election of directors, the ratification of the appointment of our auditor, the amendment of our stock plan to increase the number of shares that may be granted under the plan, and approving an amendment to our certificate of incorporation to increase our authorized shares, we are not aware of any other business to be acted upon at the annual meeting. If you grant a proxy, the person named as proxy holder, Sandesh Seth, our Chairman and Chief Executive Officer, or CEO, will have the discretion to vote your shares on any additional matters properly presented for a vote at the annual meeting.
What happens if I do not give specific voting instructions?
If you hold shares in your name and you sign and return a proxy card without giving specific voting instructions, your shares will be voted as recommended by our Board of Directors, or Board, on all matters and as the proxy holder may determine in his discretion with respect to any other matters properly presented for a vote before the annual meeting. If you hold your shares through a stockbroker, bank or other nominee and you do not provide instructions on how to vote, your stockbroker or other nominee may exercise their discretionary voting power with respect to certain proposals that are considered as “routine” matters. For example, Proposal 2 — ratification of the appointment of Marcum LLP as our independent registered public accounting firm, and Proposal 4 — the amendment to our certificate of incorporation to increase our authorized shares, are considered routine matters, and thus your stockbroker, bank or other nominee may exercise their discretionary voting power with respect to Proposals 2 and 4. If the organization that holds your shares does not receive instructions from you on how to vote your shares on a non-routine matter, the organization that holds your shares will inform us that it does not have the authority to vote on these matters with respect to your shares. This is generally referred to as a “broker non-vote.” When the vote is tabulated for any particular matter, broker non-votes will be counted for purposes of determining whether a quorum is present, but will not otherwise be counted. In the absence of specific instructions from you, your broker does not have discretionary authority to vote your shares with respect to Proposal 1 — the election of Sandesh Seth and Jeffrey W. Chell, M.D. as members to our Board, and Proposal 3 — the increase in shares of our stock plan. We encourage you to provide voting instructions to the organization that holds your shares by carefully following the instructions provided in the notice.
| 2 |
What is the quorum requirement for the annual meeting?
On October 24, 2018, the Record Date for determining which stockholders are entitled to vote, there were 114,698,044 shares of our common stock outstanding, which is our only class of voting securities. Each share of common stock entitles the holder to one vote on matters submitted to a vote of our stockholders. Thirty Four percent (34%) of our outstanding common shares as of the Record Date must be present at the annual meeting (in person or represented by proxy) in order to hold the meeting and conduct business. This is called a quorum. Your shares will be counted for purposes of determining if there is a quorum, even if you wish to abstain from voting on some or all matters introduced at the annual meeting, if you are present and vote in person at the meeting or have properly submitted a proxy card or voted by fax, by phone or by using the Internet.
How can I change my vote after I return my proxy card?
You may revoke your proxy and change your vote at any time before the final vote at the annual meeting. You may do this by signing a new proxy card with a later date, by voting on a later date by using the Internet (only your latest Internet proxy submitted prior to the annual meeting will be counted), or by attending the annual meeting and voting in person. However, your attendance at the annual meeting will not automatically revoke your proxy unless you vote at the annual meeting or specifically request in writing that your prior proxy be revoked.
Is my vote confidential?
Proxy instructions, ballots and voting tabulations that identify individual stockholders are handled in a manner that protects your voting privacy. Your vote will not be disclosed either within our company or to third parties, except:
as necessary to meet applicable legal requirements;
to allow for the tabulation of votes and certification of the vote; and
to facilitate a successful proxy solicitation.
Any written comments that a stockholder might include on the proxy card will be forwarded to our management.
Where can I find the voting results of the annual meeting?
The preliminary voting results will be announced at the annual meeting. The final voting results will be tallied by our Inspector of Elections and reported in a Current Report on Form 8-K which we will file with the Securities and Exchange Commission, or SEC, within four business days of the date of the annual meeting.
How can I obtain a separate set of voting materials?
To reduce the expense of delivering duplicate voting materials to our stockholders who may have more than one Actinium Pharmaceuticals, Inc. stock account, we are delivering only one Notice to certain stockholders who share an address, unless otherwise requested. If you share an address with another stockholder and have received only one Notice, you may write or call us to request to receive a separate Notice. Similarly, if you share an address with another stockholder and have received multiple copies of the Notice, you may write or call us at the address and phone number below to request delivery of a single copy of this Notice. For future annual meetings, you may request separate Notices, or request that we send only one Notice to you if you are receiving multiple copies, by writing or calling us at:
Actinium Pharmaceuticals, Inc.
Attention: Steve O’Loughlin, Principal Financial Officer
275 Madison Avenue, 7th Floor
New York, New York 10016
Tel: (646) 677-3875
| 3 |
Who pays for the cost of this proxy solicitation?
We will pay the costs of the solicitation of proxies. We may also reimburse brokerage firms and other persons representing beneficial owners of shares for expenses incurred in forwarding the voting materials to their customers who are beneficial owners and obtaining their voting instructions. In addition to soliciting proxies by mail, our board members, officers and employees may solicit proxies on our behalf, without additional compensation, personally, electronically or by telephone.
How can I obtain a copy of Actinium Pharmaceuticals, Inc.’s 2017 Annual Report on Form 10-K?
This proxy statement and our 2017 annual report to stockholders are available for viewing, printing and downloading at www.proxyvote.com. To view these materials, please have your 12-digit control number(s) available that appears on your Notice or proxy card. On this website, you can also elect to receive future distributions of our proxy statements and annual reports to stockholders by electronic delivery.
Additionally, you can find a copy of our Annual Report on Form 10-K, which includes our financial statements, for the fiscal year ended December 31, 2017 on the website of the SEC, at www.sec.gov, or in the “All SEC Filings” section of the “Investors” section of our website at www.actiniumpharma.com. You may also obtain a printed copy of our Annual Report on Form 10-K including our financial statements, free of charge, from us by sending a written request to: Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York, NY 10016, attention: Principal Financial Officer.
What is the voting requirement to elect directors?
Directors are elected by a plurality of the votes cast in person or by proxy at the annual meeting and entitled to vote on the election of directors. “Plurality” means that the nominees receiving the greatest number of affirmative votes will be elected as directors, up to the number of directors to be chosen at the meeting. Broker non-votes will not affect the outcome of the election of directors because brokers do not have discretion to cast votes on this proposal without instruction from the beneficial owner of the shares.
What is the voting requirement to approve the other three proposals?
The proposal to ratify the appointment of Marcum LLP as our independent registered public accounting firm will be approved if there is a quorum and the votes cast “FOR” the proposal exceeds those cast against the proposal. The proposal to approve an amendment to our 2013 stock plan to increase the shares authorized under the plan will be approved if there is a quorum and the votes cast “FOR” the proposal exceeds those cast against the proposal. The proposal to amend the charter to increase the authorized shares of the company will be approved if at least 50% of the issued and outstanding shares votes “FOR” the proposal and this exceeds those cast against the proposal.
Abstentions and broker non-votes will be treated as shares that are present, or represented and entitled to vote for purposes of determining the presence of a quorum at the annual meeting. Abstentions will not be counted in determining the number of votes cast in connection with any matter presented at the annual meeting. Broker non-votes will not be counted as a vote cast on any matter presented at the annual meeting.
Do I Have Dissenters’ (Appraisal) Rights?
Appraisal rights are not available to our shareholders with any of the proposals described above to be brought before the annual meeting of shareholders.
How can I communicate with the non-employee directors on the Actinium Pharmaceuticals, Inc. Board of Directors?
Our Board encourages stockholders who are interested in communicating directly with the non-employee directors as a group to do so by writing to the non-employee directors in care of our Chairman and CEO. Stockholders can send communications by mail to:
Sandesh Seth, Chairman and Chief Executive
Officer
Actinium Pharmaceuticals, Inc.
275 Madison Avenue, 7th Floor
New York, New York 10016
Correspondence received that is addressed to the non-employee directors will be reviewed by our Chairman of the Board or his designee, who will regularly forward to the non-employee directors a summary of all such correspondence and copies of all correspondence that, in the opinion of our chairman, deals with the functions of our Board or committees thereof or that our chairman otherwise determines requires their attention. Directors may at any time review a log of all correspondence received by us that is addressed to the non-employee members of our Board and request copies of any such correspondence.
| 4 |
WHO CAN HELP ANSWER YOUR QUESTIONS?
You may seek answers to your questions by writing, calling or emailing us at:
Steve O’Loughlin
Principal Financial Officer
Actinium Pharmaceuticals, Inc.
275 Madison Avenue, 7th Floor
New York, NY 10016
Email: soloughlin@actiniumpharma.com
Tel: 646-677-3875
CORPORATE GOVERNANCE
Board of Directors
The Board of Directors oversees our business affairs and monitors the performance of management. In accordance with our corporate governance principles, our Board does not involve itself in day-to-day operations. The directors keep themselves informed through discussions with the Chairman and CEO, other key executives, and by reading the reports and other materials that we send them and by participating in Board and committee meetings. Our directors hold office until their successors have been elected and duly qualified unless the director resigns or by reason of death or other cause is unable to serve in the capacity of director. Biographical information about our directors is provided in “Election of Directors — Proposal No. 1” on page 29.
Term of Office
Our directors are divided into three classes, designated Class I, Class II and Class III. Class I shall consists of two directors, Class II shall consists of two directors, and Class III consists of one director.
The term of each director is set forth below or until their successors are duly elected:
| Director | Class | Term (from 2018 Annual Meeting, if elected) | ||
| David Nicholson | Class I | 2 years | ||
| Richard I Steinhart | Class I | 2 years | ||
| Sandesh Seth | Class II | 3 years | ||
| Jeffrey W. Chell | Class II | 3 years | ||
| Ajit S. Shetty | Class III | 1 year |
Notwithstanding the foregoing, each director shall serve until his successor is duly elected and qualified, or until his retirement, death, resignation or removal. In order to implement a classified board of directors, Class I serves a two year term from the date of the 2018 Annual Shareholders Meeting; Class II serves a three year term from the date of the 2018 Annual Shareholders Meeting; and Class III serves a one year term from the date the date of the 2018 Annual Shareholders Meeting. Directors elected at each annual meeting are elected for a three year term.
Director Independence
We use the definition of “independence” of the NYSE American stock exchange to make this determination. We are listed on the NYSE American under the symbol “ATNM”. NYSE MKT corporate governance rule Sec. 803(A)(2) provides that an “independent director” means a person other than an executive officer or employee of the company. No director qualifies as independent unless the issuer’s board of directors affirmatively determines that the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The following is a non-exclusive list of persons who shall not be considered independent under NYSE American rules:
a director who is, or during the past three years was, employed by the company, other than prior employment as an interim executive officer (provided the interim employment did not last longer than one year);
| 5 |
a director who accepted or has an immediate family member who accepted any compensation from the company in excess of $120,000 during any period of twelve consecutive months within the three years preceding the determination of independence, other than the following:
| (i) | compensation for board or board committee service; |
| (ii) | compensation paid to an immediate family member who is an employee (other than an executive officer) of the company, |
| (iii) | compensation received for former service as an interim executive officer (provided the interim employment did not last longer than one year); or |
| (iv) | benefits under a tax-qualified retirement plan, or non-discretionary compensation; |
a director who is an immediate family member of an individual who is, or at any time during the past three years was, employed by the company as an executive officer;
a director who is, or has an immediate family member who is, a partner in, or a controlling shareholder or an executive officer of, any organization to which the company made, or from which the company received, payments (other than those arising solely from investments in the company’s securities or payments under non-discretionary charitable contribution matching programs) that exceed 5% of the organization’s consolidated gross revenues for that year, or $200,000, whichever is more, in any of the most recent three fiscal years;
a director who is, or has an immediate family member who is, employed as an executive officer of another entity where at any time during the most recent three fiscal years any of the issuer’s executive officers serve on the compensation committee of such other entity; or
a director who is, or has an immediate family member who is, a current partner of the company’s outside auditor, or was a partner or employee of the company’s outside auditor who worked on the company’s audit at any time during any of the past three years.
Under the above-mentioned NYSE American director independence rules, Jeffrey W. Chell, David Nicholson, Ajit S. Shetty, and Richard I. Steinhart are independent directors of the Company.
Board Leadership Structure
In October 2013, Sandesh Seth was appointed Chairman of our Board of Directors and in June 2017 Mr. Seth was named Chief Executive Officer of the Company. In September 2017, David Nicholson was appointed lead independent director of our board of directors (“Lead Independent Director”). Our Lead Independent Director chairs the executive sessions of our board of director meetings; provides feedback to the Chairman and CEO; if appropriate, and in coordination with executive management, be available for consultation and direct communication with major shareholders; and leads the board’s evaluation of the Chairman and CEO. We have a separate chair for each committee of our board of directors, all of whom are independent directors. The chairs of each committee report on the activities of their committees in fulfilling their responsibilities at the meetings of our board of directors.
Board of Directors Meetings and Attendance
During the fiscal year 2017, our Board held 15 meetings. No director attended fewer than 93% of the total number of meetings of our Board and of any committees of which he was a member during the year ended December 31, 2017. It is our policy that directors should make every effort to attend the annual meeting of stockholders, and each of our directors attended the annual meeting of stockholders in 2017.
| 6 |
Code of Business Conduct and Ethics
We adopted a Code of Business Conduct and Ethics that applies to all of our directors, officers and employees, including our principal executive officer and principal financial and accounting officer. A copy of the Code of Business Conduct and Ethics is available on the Investor section of our website at www.actiniumpharma.com. We will post on our website any amendment to our Code of Business Conduct and Ethics or waivers of our Code of Business Conduct and Ethics for directors and executive officers.
Complaints Regarding Accounting Matters
The Audit Committee has established procedures for:
the receipt, retention and treatment of complaints regarding accounting, internal accounting controls, or auditing matters; and
the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters.
Communications with Directors
The Board of Directors has approved procedures for stockholders to send communications to individual directors or the non-employee directors as a group. Written correspondence should be addressed to the director or directors in care of Sandesh Seth, Chairman and CEO of Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York, NY 10016. Correspondence received that is addressed to the non-employee directors will be reviewed by our Chairman and CEO or his designee, who will regularly forward to the non-employee directors a summary of all such correspondence and copies of all correspondence that, deals with the functions of our Board of Directors or committees thereof or that he otherwise determines requires their attention. Directors may at any time review a log of all correspondence received by us that is addressed to the non-employee members of our Board of Directors and request copies of any such correspondence. You may also contact individual directors by calling our principal executive offices at (646) 677-3875.
Legal Proceedings
There are no legal proceedings to which any director, director nominee or officer of our company, any owner of record or beneficially of more than 5% of common stock, or any associate of any such director, director nominee, officer of our company or major security holder is a party in legal proceedings adverse to our company or has a material interest adverse to us.
Compliance With Section 16(a) of the Exchange Act
Based solely upon a review of copies of such forms filed on Forms 3, 4, and 5, and amendments thereto furnished to us we believe that as of the date of this proxy, our executive officers, directors and greater than 10 percent beneficial owners have complied on a timely basis with all Section 16(a) filing requirements, with the exception of a Form 4 filed by Dr. Berger on March 19, 2018 for a transaction on March 6, 2018.
BOARD COMMITTEES
Committees of the Board of Directors
Our Board of Directors has formed three standing committees: audit, compensation and corporate governance. Actions taken by our committees are reported to the full board. Each of our committees has a charter and each charter is posted on our website.
| Audit Committee | Compensation Committee | Corporate Governance Committee | ||
| Richard I. Steinhart* | David Nicholson* | Ajit S. Shetty* | ||
| Jeffrey W. Chell | Jeffrey W. Chell | David Nicholson | ||
| Ajit S. Shetty | Ajit S. Shetty | Richard I. Steinhart |
| * | Indicates committee chair |
| 7 |
Audit Committee
Our audit committee, which currently consists of three directors, provides assistance to our board in fulfilling its legal and fiduciary obligations with respect to matters involving the accounting, financial reporting, internal control and compliance functions of the company. Our audit committee employs an independent registered public accounting firm to audit the financial statements of the company and perform other assigned duties. Further, our audit committee provides general oversight with respect to the accounting principles employed in financial reporting and the adequacy of our internal controls. In discharging its responsibilities, our audit committee may rely on the reports, findings and representations of the company’s auditors, legal counsel, and responsible officers. Our board has determined that all members of the audit committee are financially literate within the meaning of SEC rules and under the current listing standards of the NYSE MKT. Richard I. Steinhart is the chairman of the audit committee. Our audit committee met four times during the year ended December 31, 2017.
Compensation Committee
Our compensation committee, which currently consists of three directors, establishes executive compensation policies consistent with the company’s objectives and stockholder interests. Our compensation committee also reviews the performance of our executive officers and establishes, adjusts and awards compensation, including incentive-based compensation, as more fully discussed below. Our compensation committee met two times during the year ended December 31, 2017.
In addition, our compensation committee generally is responsible for:
establishing and periodically reviewing our compensation philosophy and the adequacy of compensation plans and programs for our directors, executive officers and other employees;
overseeing our compensation plans, including the establishment of performance goals under the company’s incentive compensation arrangements and the review of performance against those goals in determining incentive award payouts;
overseeing our executive employment contracts, special retirement benefits, severance, change in control arrangements and/or similar plans;
acting as administrator of any company stock option plans; and
overseeing the outside consultant, if any, engaged by the compensation committee.
Our compensation committee periodically reviews the compensation paid to our non-employee directors and the principles upon which their compensation is determined. The compensation committee also periodically reports to the board on how our non-employee director compensation practices compare with those of other similarly situated public corporations and, if the compensation committee deems it appropriate, recommends changes to our director compensation practices to our board for approval.
Outside consulting firms retained by our compensation committee and management also will, if requested, provide assistance to the compensation committee in making its compensation-related decisions.
Corporate Governance Committee
Our corporate governance committee, which currently consists of three directors, monitors our corporate governance system. Our corporate governance committee met two times during the year ended December 31, 2017.
Nomination of Directors
Board of Director nominations are selected, or recommended for our Board’s selection, by a majority of the independent directors. Our independent directors include Jeffrey W. Chell, David Nicholson, Ajit S. Shetty and Richard I. Steinhart. These directors are charged with the responsibility of proposing potential director nominees to the board of directors for consideration. All of our independent directors are independent directors as defined by the rules of the NYSE MKT. Our independent directors use criteria by which they will seek to evaluate candidates to serve on our Board. The evaluation methodology includes items such as experience in the biotechnology sector, experience with public companies, executive managerial experience, operations and commercial experience, fundraising experience and contacts in the investment banking industry, personal and skill set compatibility with current board members, industry reputation, knowledge of our company generally, and independence.
| 8 |
DIRECTOR COMPENSATION
The following table sets forth the compensation of our non-employee directors for the 2017 fiscal year:
| Name | Fees Earned or Paid in Cash | Stock Awards | Option (1) | All Other Compensation | Total | |||||||||||||||
| David Nicholson (2) | $ | 59,000 | - | 73,430 | - | $ | 132,430 | |||||||||||||
| Ajit J. Shetty (3) | 39,694 | - | 82,903 | - | $ | 122,597 | ||||||||||||||
| Richard Steinhart | $ | 63,000 | - | 73,430 | - | $ | 136,430 | |||||||||||||
| Sergio Traversa (4) | $ | 58,500 | - | 73,430 | - | $ | 131,930 | |||||||||||||
| (1) | At the end of fiscal year 2017, the aggregate number of option awards outstanding for each director was as follows: (i) for Dr. Nicholson, 274,900, (ii) for Dr. Shetty, 75,000, (iii) for Mr. Steinhart, 224,950, and (iv) for Mr. Traversa, 172,950. |
| (2) | Mr. Nicholson was named Lead Director in September 2017 and receives an additional $10,000 per year for his role as Lead Director. |
| (3) | Dr. Shetty was appointed a director on March 28, 2017. |
| (4) | Mr. Traversa resigned from the company on June 6, 2017. |
In accordance with SEC rules, the amounts shown reflect the aggregate grant date fair value of option awards granted to Non-Employee Directors during 2017, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718.
Our non-employee directors are paid an annual fee of $40,000 and receive annual option grants. Board committee members will receive the following compensation:
| BOD Committee | Chairman | Member | ||||||
| Audit | $ | 20,000 | $ | 6,000 | ||||
| Compensation | $ | 10,000 | $ | 5,000 | ||||
| Corporate Governance | $ | 7,500 | $ | 3,000 | ||||
AUDIT COMMITTEE REPORT
Report of the Audit Committee of the Board of Directors
The Audit Committee provides assistance to the Board of Directors in fulfilling its oversight responsibilities relating to our corporate accounting and reporting practices toward assurance of the quality and integrity of our consolidated financial statements. The purpose of the Audit Committee is to serve as an independent and objective party to monitor our financial reporting process and internal control system; oversee, review and appraise the audit activities of our independent registered public accounting firm and internal auditing function, maintain complete, objective and open communication between the Board of Directors, the independent accountants, financial management and the internal audit function.
Our independent registered public accounting firm reports directly to the Audit Committee and the Audit Committee is solely responsible to appoint or replace our independent registered public accounting firm and to assure its independence and to provide oversight and supervision thereof. The Audit Committee determines compensation of the independent registered public accounting firm and has established a policy for approval of non-audit related engagements awarded to the independent registered public accounting firm. Such engagements must not impair the independence of the registered public accounting firm with respect to our company as prescribed by the Sarbanes-Oxley Act of 2002; thus payment amounts are limited and non-audit related engagements must be approved in advance by the Audit Committee. The Audit Committee determines the extent of funding that we must provide to the Audit Committee to carry out its duties and has determined that such amounts were sufficient in 2017.
| 9 |
With respect to the fiscal year ended December 31, 2017, in addition to its other work, the Audit Committee:
Reviewed and discussed with management our audited consolidated financial statements as of December 31, 2017 and for the year then ended; and
Discussed with GBH CPAs, PC the matters required to be discussed by Statement on Auditing Standards No. 61, “Communication with Audit Committees,” as amended, with respect to its review of the findings of the independent registered public accounting firm during its examination of our financial statements.
The Audit Committee recommended, based on the review and discussion summarized above, that the Board of Directors include the 2017 audited consolidated financial statements in the 2017 Form 10-K for the fiscal year ended December 31, 2017 for filing with the SEC.
| Audit Committee of the Board of Directors of Actinium Pharmaceuticals, Inc. | ||
Richard I. Steinhart, Chairman Jeffrey W. Chell Ajit S. Shetty | ||
Information About Our Auditors
Our Audit Committee of our Board appointed GBH CPAs, PC as the independent registered public accounting firm to conduct the audit of our consolidated financial statements for the 2017 fiscal year and to report on our consolidated balance sheets, statements of income and other related statements. GBH CPAs, PC served as our independent registered public accounting firm since December 2012. In August 2018, we appointed Marcum LLP as our independent registered public accounting firm due to a merger between GBH CPAs, PC and Marcum LLP. The Audit Committee Charter includes the procedures for pre-approval of all fees charged by our independent registered public accounting firm. Under the procedure, our Audit Committee approves the engagement letter with respect to audit and review services. Other fees are subject to pre-approval by our Audit Committee. The audit and audit-related fees paid to the auditors with respect to the 2017 fiscal year were pre-approved by our Audit Committee.
Fees and Services
The aggregate fees billed for the fiscal years ended December 31, 2017 and 2016, respectively, for professional services rendered by GBH CPAs, PC for the audits of the Company’s annual financial statements included in Form 10-K, or Audit Fees, tax compliance, advice, and planning, or Tax Fees, and all other fees:
| Year Ended December 31, 2017 | Year Ended December 31, 2016 | |||||||
| Audit Fees | $ | 116,500 | $ | 123,168 | ||||
| Audit – Related Fees | 60,800 | 23,750 | ||||||
| Tax Fees | - | - | ||||||
| All Other Fees | - | - | ||||||
| Total | $ | 177,300 | $ | 146,918 | ||||
Pre-Approval Policy
In 2015, the Audit Committee adopted policies and procedures for the pre-approval of audit and non-audit services performed by the independent registered public accountants pursuant to which the Audit Committee generally is required to pre-approve the audit and permissible non-audit services performed by the independent registered public accountants in order to ensure that the provision of such services does not impair the registered accountants’ independence.
| 10 |
Compensation Committee Report*
Our Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis (“CD&A”) included in this proxy statement. Based on that review and discussion, the Compensation Committee has recommended to our Board that the CD&A be included in the proxy statement.
Submitted by:
The Compensation Committee of the Board of Directors
/s/ David Nicholson, Chairman
/s/ Jeffrey W. Chell
/s/ Ajit S. Shetty
| * | The information contained in this Compensation Committee Report shall not be deemed to be “soliciting material” or “filed” or incorporated by reference in future filings with the SEC, or subject to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), except to the extent that we specifically request that the information be treated as soliciting material or specifically incorporate it by reference into a document filed under the Securities Act of 1933, as amended, or the Exchange Act. |
Compensation Discussion and Analysis
Our Compensation Committee of our Board of Directors has the responsibility to review, determine and approve the compensation for our executive officers. Further, our Compensation Committee oversees our overall compensation strategy, including compensation policies, plans and programs that cover all employees. In 2016, our Stockholders voted on an advisory basis with respect to our compensation program for named executive officers. Of the votes cast (excluding abstentions and broker non-votes), 69.0% were cast in support of the program. In light of this, in reviewing the executive compensation program for 2016, our Compensation Committee decided to retain the general overall program design, which ties a significant portion of the executives’ pay closely with our performance. In the future, our Compensation Committee will continue to consider the executive compensation program in light of changing circumstances and stockholder feedback.
We currently employ six executive officers, each of whom serves as a “Named Executive Officer” (or NEO) for purposes of SEC reporting: (1) Sandesh Seth, our Chairman and Chief Executive Officer (who we refer to in this Compensation Discussion and Analysis as our CEO); (2) Steve O’Loughlin, our Principal Financial Officer, (3) Mark Berger, our Chief Medical Officer; (4) Nitya Ray, our Executive Vice-President, Head of Product Development, Manufacturing and Supply Chain; (5) Anil Kapur, our Chief Commercial Officer; and (6) Dale Ludwig, our Chief Scientific Officer.
This Compensation Discussion and Analysis sets forth a discussion of the compensation for our NEOs as well as a discussion of our philosophies underlying the compensation for our NEOs and our employees generally.
Objectives of Our Compensation Program
The Compensation Committee’s philosophy seeks to align the interests of our stockholders, officers and employees by tying compensation to individual and company performance, both directly in the form of salary or annual cash incentive payments, and indirectly in the form of equity awards. The objectives of our compensation program enhance our ability to:
attract and retain qualified and talented individuals; and
provide reasonable and appropriate incentives and rewards to our team for building long-term value within our company, in each case in a manner comparable to companies similar to ours.
In addition, we strive to be competitive with other similarly situated companies in our industry. The process of developing pharmaceutical products and bringing those products to market is a long-term proposition and outcomes may not be measurable for several years. Therefore, in order to build long-term value for our company and its stockholders, and in order to achieve our business objectives, we believe that we must compensate our officers and employees in a competitive and fair manner that reflects current company activities, but also reflects contributions to building long-term value.
| 11 |
We utilize the services of StreeterWyatt Governance LLC to review compensation programs of peer companies in order to assist the Compensation Committee in determining the compensation levels for our NEOs, as well as for other employees of our company. StreeterWyatt is a recognized independent consulting company and services clients throughout the United States.
The companies that comprise our peer group are selected and reviewed no less frequently than biennially.
Elements of Our Compensation Program and Why We Chose Each
Main Compensation Components
Our company-wide compensation program, including for our NEOs, is broken down into three main components: base salary, performance cash bonuses and potential long-term compensation in the form of stock options or restricted stock awards. We believe these three components constitute the minimum essential elements of a competitive compensation package in our industry.
Salary
Base salary is used to recognize the experience, skills, knowledge and responsibilities required of our NEOs as well as recognizing the competitive nature of the biopharmaceutical industry. This is determined partially by evaluating our peer companies as well as the degree of responsibility and experience levels of our NEOs and their overall contributions to our company. Base salary is one component of the compensation package for NEOs; the other components being cash bonuses, annual equity grants, and company benefit programs. Base salary is determined in advance whereas the other components of compensation are awarded in varying degrees following an assessment of the performance of a NEO. This approach to compensation reflects the philosophy of our Board and its Compensation Committee to emphasize and reward, on an annual basis, performance levels achieved by our NEOs.
Performance Bonus Plan
We have a performance bonus plan under which bonuses are paid to our NEOs based on achievement of company performance goals and objectives established by the Compensation Committee and/or our Board as well as on individual performance. The bonus program is discretionary and is intended to: (i) strengthen the connection between individual compensation and our company’s achievements; (ii) encourage teamwork among all disciplines within our company; (iii) reinforce our pay-for-performance philosophy by awarding higher bonuses to higher performing employees; and (iv) help ensure that our cash compensation is competitive. Depending on the cash position of the company, the Compensation Committee and our Board have the discretion to not pay cash bonuses in order that we may conserve cash and support ongoing development programs and commercialization efforts. Regardless of our cash position, we consistently grant annual merit-based stock options to continue incentivizing both our senior management and our employees.
Based on their employment agreements, each NEO is assigned a target payout under the performance bonus plan, expressed as a percentage of base salary for the year. Actual payouts under the performance bonus plan are based on the achievement of corporate performance goals and an assessment of individual performance, each of which is separately weighted as a component of such officer’s target payout. For the NEOs, the corporate goals receive the highest weighting in order to ensure that the bonus system for our management team is closely tied to our corporate performance. Each employee also has specific individual goals and objectives as well that are tied to the overall corporate goals. For employees, mid-year and end-of-year progress is reviewed with the employees’ managers.
Equity Incentive Compensation
We view long-term compensation, currently in the form of stock options and restricted stock generally vesting in annual increments over four years, as a tool to align the interests of our NEOs and employees generally with the creation of stockholder value, to motivate our employees to achieve and exceed corporate and individual objectives and to encourage them to remain employed by the company. While cash compensation is a significant component of employees’ overall compensation, the Compensation Committee and our Board (as well as our NEOs) believe that the driving force of any employee working in a small biotechnology company should be strong equity participation. We believe that this not only creates the potential for substantial longer term corporate value but also serves to motivate employees and retain their loyalty and commitment with appropriate personal compensation.
| 12 |
Other Compensation
In addition to the main components of compensation outlined above, we also provide contractual severance and/or change in control benefits to our Chairman and CEO. The change in control benefits for all applicable persons have a “double trigger.” A double trigger means that the executive officers will receive the change in control benefits described in the agreements only if there is both (1) a Change in Control of our company (as defined in the agreements) and (2) a termination by us of the applicable person’s employment “without cause” or a resignation by the applicable persons for “good reason” (as defined in the agreements) within a specified time period prior to or following the Change in Control. We believe this double trigger requirement creates the potential to maximize stockholder value because it prevents an unintended windfall to management, as no benefits are triggered solely in the event of a Change in Control, while providing appropriate incentives to act in furtherance of a change in control that may be in the best interests of the stockholders. We believe these severance or change in control benefits are important elements of our compensation program that assist us in retaining talented individuals at the executive and senior managerial levels and that these arrangements help to promote stability and continuity of our executives and senior management team. Further, we believe that the interests of our stockholders will be best served if the interests of these members of our management are aligned with theirs. We believe that providing change in control benefits lessens or eliminates any potential reluctance of members of our management to pursue potential change in control transactions that may be in the best interests of the stockholders. We also believe that it is important to provide severance benefits to members of our management, to promote stability and focus on the job at hand.
We also provide benefits to the executive officers that are generally available to all regular full-time employees of our company, including our medical and dental insurance, and a 401(k) plan. At this time, we do not provide any perquisites to any of our NEOs. Further, we do not have deferred compensation plans, pension arrangements or post-retirement health coverage for our executive officers or employees. All of our employees not specifically under contract are “at-will” employees, which means that their employment can be terminated at any time for any reason by either us or the employee. Our Chairman and CEO has an employment agreement that provides lump sum compensation in the event of his termination without cause or, under certain circumstances, upon a Change of Control.
Determination of Compensation Amounts
A number of factors impact the determination of compensation amounts for our NEOs, including the individual’s role in the company and individual performance, length of service with the company, competition for talent, individual compensation package, assessments of internal pay equity and industry data. Stock price performance has generally not been a factor in determining annual compensation because the price of our common stock is subject to a variety of factors outside of our control.
Industry Survey Data
In collaboration with StreeterWyatt, we establish and maintain a list of peer companies to best assure ourselves that we are compensating our executives on a fair and reasonable basis, as set forth above under the heading “Objectives of our Compensation Program.” We also utilize StreetWyatt-prepared data for below-executive level personnel, which data focuses on similarly-sized bio-tech companies. The availability of peer data is used by the Compensation Committee strictly as a guide in determining compensation levels with regard to salaries, cash bonuses and performance-related annual equity grants to all employees. However, the availability of this data does not imply that the Compensation Committee is under any obligation to exactly follow peer companies in compensation matters.
Determination of Base Salaries
As a guideline for NEO base salary, we perform formal benchmarks against respective comparable positions in our established peer group. We adjust salaries based on our assessment of our NEOs’ levels of responsibility, experience, overall compensation structure and individual performance. The Compensation Committee is not obliged to raise salaries purely on the availability of data. Merit-based increases to salaries of executive officers are based on our assessment of individual performance and the relationship to applicable salary ranges. Cost of living adjustments may also be a part of that assessment.
| 13 |
Performance Bonus Plan
Concurrently with the beginning of each calendar year, preliminary corporate goals that reflect our business priorities for the coming year are prepared by the CEO with input from the other executive officers. These goals are weighted by relative importance. The draft goals and proposed weightings are presented to the Compensation Committee and the Board and discussed, revised as necessary, and then approved by our Board. The Compensation Committee then reviews the final goals and their weightings to determine and confirm their appropriateness for use as performance measurements for purposes of the bonus program. The goals and/or weightings may be re-visited during the year and potentially restated in the event of significant changes in corporate strategy or the occurrence of significant corporate events. Following the agreement of our Board on the corporate objectives, the goals are then shared with all employees in a formal meeting(s), and are reviewed periodically throughout the year.
Determination of Equity Incentive Compensation
To assist us in assessing the reasonableness of our equity grant amounts, we have reviewed StreeterWyatt supplied information. Such information included equity data from a cross-section of similar companies in our industry.
Equity Grant Practices
All stock options and/or restricted stock granted to the NEOs and other executives are approved by the Compensation Committee. Exercise prices for options are set at the closing price of our common stock on the date of grant. Grants are generally made: (i) on the employee’s start date and (ii) at our Board meetings held each February and following annual performance reviews. However, grants have been made at other times during the year. The size of year-end grants for each NEO is assessed against our internal equity guidelines. Current market conditions for grants for comparable positions and internal equity may also be assessed. Also, grants may be made in connection with promotions or job-related changes in responsibilities. In addition, on occasion, the Compensation Committee may make additional special awards for extraordinary individual or company performance.
Compensation Setting Process
At the February meetings of our Board and the Compensation Committee, overall corporate performance and relative achievement of the corporate goals for the prior year are assessed. The relative achievement of each goal is assessed and quantified and the summation of the individual components results in a corporate goal rating, expressed as percentages. The Compensation Committee then approves the final disbursement of salary increases, cash bonuses and option or restricted stock grants.
The Compensation Committee looks to the CEO’s performance assessments of the other NEOs and his recommendations regarding a performance rating for each, as well as input from the other members of our Board. These recommendations may be adjusted by the Compensation Committee prior to finalization. For the CEO, the Compensation Committee evaluates his performance, taking into consideration input from the other members of our Board, and considers the achievement of overall corporate objectives by both the CEO specifically and the company generally. The CEO is not present during the Compensation Committee’s deliberations regarding his compensation.
The Compensation Committee has the authority to directly engage, at our company’s expense, any compensation consultants or other advisors (such as StreeterWyatt) that it deems necessary to determine the amount and form of employee, executive and director compensation. In determining the amount and form of employee, executive and director compensation, the Compensation Committee has reviewed and discussed historical salary information as well as salaries for similar positions at comparable companies. However the availability of this data does not imply that the Compensation Committee is under any obligation to exactly follow peer companies’ compensation practices.
We paid consultant fees to StreeterWyatt of $10,000. NEOs may have indirect input in the compensation results for other executive officers by virtue of their participation in the performance review and feedback process for the other executive officers.
| 14 |
DIRECTORS AND EXECUTIVE OFFICERS
Directors And Executive Officers
The names, positions and ages of our directors and executive officers as of October 24, 2018, are as follows:
| Name | Age | Position | ||
| Sandesh Seth | 54 | Chairman and Chief Executive Officer | ||
| Mark S. Berger, MD | 64 | Chief Medical Officer | ||
| Nitya Ray, Ph.D. | 66 | Executive Vice President, Head of Product Development, Manufacturing and Supply Chain | ||
| Anil Kapur | 49 | Chief Commercial Officer | ||
| Dale L. Ludwig, Ph. D. | 56 | Chief Scientific Officer | ||
| Steve O’Loughlin | 33 | Principal Financial Officer (Principal Financial and Accounting Officer) | ||
| Jeffrey W. Chell. M.D. | 64 | Director | ||
| David Nicholson, Ph. D. | 63 | Lead Independent Director | ||
| Ajit S. Shetty, Ph.D. | 72 | Director | ||
| Richard I. Steinhart | 61 | Director |
Subject to the classified board provisions of our charter, all directors hold office until the next annual meeting of stockholders and the election and qualification of their successors. Officers are elected annually by our Board and serve at the discretion of our Board.
There are no arrangements or understanding between any of our directors and any other persons pursuant to which they were selected as a director.
Background of Executive Officers and Directors
The principal occupations for the past five years (and, in some instances, for prior years) of each of our directors and executive officers are as follows:
Sandesh Seth, MS, MBA, Chairman and CEO
Mr. Sandesh Seth has been our Chief Executive Officer since June 2017. Mr. Seth has been a Director since March 2012, our Chairman of the Board since October 2013, and served as Executive Chairman from August 2014 to June 2017. Mr. Seth was affiliated with Laidlaw & Co. (UK) Ltd., a healthcare focused, investment banking and wealth management firm where he was Head of Healthcare Investment Banking. Mr. Seth is the Chairman of the Board of Relmada Therapeutics, Inc., a publicly listed, specialty pharmaceuticals company focused on pain therapeutics.
Mr. Seth has 25+ years of experience in investment banking (Cowen & Co.), equity research (Bear Stearns, Commonwealth Associates) and in the pharma industry (Pfizer, Warner-Lambert, SmithKline in strategic planning, business development and R&D project management). Mr. Seth has an MBA in Finance from New York University; an M.S. in the Pharmaceutical Sciences from the University of Oklahoma Health Center and a B.Sc. in Chemistry from Bombay University. He has published several scientific articles and was awarded the University Regents Award for Research Excellence at the University of Oklahoma. Mr. Seth was designated as Regulatory Affairs Certified (R.A.C.) by the Regulatory Affairs Professionals Society which signifies proficiency with U.S. FDA regulations.
That Mr. Seth has served in various business executive-level positions over the course of his career, has significant investment banking experience, has developed significant management and leadership skills and is well accustomed to interfacing with investors, analysts, auditors, C-level executives, and outside advisors, led us to conclude that Mr. Seth should serve as a director.
| 15 |
Steve O’Loughlin, BS, Principal Financial Officer
Steve O’Loughlin has been our Principal Financial Officer since May 2017. Mr. O’Loughlin joined Actinium in October 2015 as Vice President, Finance and Corporate Development, with almost a decade of life sciences industry experience gained from previous positions in investment banking and publicly traded life sciences companies. Prior to Actinium, from June 2015 to October 2015, Mr. O’Loughlin worked at J. Streicher LLC as an investment banker, from August 2012 to June 2015 Mr. O’Loughlin held the position of Vice President, Corporate Finance and Development and was a corporate officer at Protea Biosciences, Inc., a publicly traded life sciences tools company. Previously, From June 2010 to June 2012, Mr. O’Loughlin held corporate development positions with Caliber I.D., a publicly traded diagnostics company. Mr. O’Loughlin previously worked in investment banking at Jesup & Lamont where he focused on the biotechnology and life sciences industries. Mr. O’Loughlin has a B.S. in Business Administration with a concentration in finance from Ramapo College of New Jersey.
Mark S. Berger, MD, Chief Medical Officer
Dr. Berger has been our Chief Medical Officer since January 2017. From September 2013 to January 2017 Dr. Berger worked for Kadmon Corporation where he was Senior Vice President, Clinical Research. In this role he was responsible for all clinical aspects of new drug development including designing and managing clinical trials in oncology indications (non-small cell lung cancer and glioblastoma) and non-oncology indications (chronic graft versus host disease and polycystic kidney disease). Dr. Berger joined Kadmon after serving as Chief Medical Officer of Deciphera Pharmaceuticals from June 2011 to September 2013. Prior to Deciphera, Dr. Berger was Vice President for Clinical Development at Gemin X Pharmaceuticals where he led the clinical strategy, design and management of clinical trials for two novel oncology agents including obatoclax, a pan Bcl-2 inhibitor. Based on the results of a randomized Phase 2 clinical trial of obatoclax, Gemin X was acquired by Cephalon in March of 2011 for a total consideration of $525 million including $225 million in an upfront cash payment.
Before his work with biotechnology companies, Dr. Berger held key positions in two global pharmaceutical companies. Dr. Berger previously served as Group Director, Medicine Development Centre-Oncology for GlaxoSmithKline. In this position Dr. Berger managed the development of Tykerb (lapatinib) in lung and breast cancer where he designed and led two Phase 2 clinical trials before planning and leading a 399 patient pivotal Phase 3 trial that resulted in the FDA approval of Tykerb in breast cancer. In addition, he managed the Lapatinib Expanded Access Program (LEAP) that enrolled over 4000 patients on a global basis. Dr. Berger began his career in drug development at Wyeth Research where he led the planning and execution of the pivotal Phase 2 trial for Mylotarg, which was the first antibody targeted chemotherapy agent and targeted CD33, similar to Actimab-A. He presented the Mylotarg clinical data at the FDA’s Oncology Drug Advisory Committee meeting, after which Mylotarg received accelerated FDA approval for patients with relapsed AML.
Dr. Berger has a B.A. in biology from Wesleyan University and received his M.D. from the University of Virginia School of Medicine. He did his Hematology-Oncology fellowship at the University of Pennsylvania where he was an Assistant Professor of Medicine, and also was a Research Fellow at the Ludwig Institute for Cancer Research and the Imperial Cancer Research Fund, both in London. Dr. Berger is board certified in internal medicine, hematology and medical oncology.
Nitya Ray, PhD, Executive Vice-President, Head of Product Development, Manufacturing and Supply Chain
Dr. Nitya Ray has been our Executive Vice-President, Head of Product Development, Manufacturing and Supply Chain since June 2017. Dr. Ray joins the Company from CytoDyn, Inc. where he was Sr. Vice President of Manufacturing and CMC Team Leader. At CytoDyn Dr. Ray developed robust and cost effective manufacturing processes for an antibody therapeutic drug candidate, currently in two Phase 3 clinical studies intended to treat and prevent HIV infection. Dr. Ray led a successful regulatory meetings with the FDA while simultaneously developing strategies for process development, scale-up, validation, commercial manufacturing, and supply chain to support potential commercialization of the CytoDyn’s HIV therapeutic drug candidate. Prior to CytoDyn, Dr. Ray spent 15 years at Progenics Pharmaceuticals, Inc., a radiopharmaceutical therapeutic and diagnostic company, most recently as Senior Vice President, Manufacturing. At Progenics, Dr. Ray led the development of scalable manufacturing processes and achieved order-of-magnitude cost reduction through improved productivity and scale. In addition, Dr. Ray built process and product development teams for Progenics’ small molecule, biologics, and radiopharmaceuticals that developed innovative processes for various phases of clinical development and commercial manufacturing. Dr. Ray supported in-house cGMP biologics manufacturing for Phase 1–3 clinical development and managed relationships with Contract Development and manufacturing Organizations (CDMO’s) on a global basis. Dr. Ray also worked at Hoffman-La Roche with a focus on biopharmaceuticals and Verax Corporation in roles of increasing responsibility. Dr. Ray has a Ph.D. in Biochemical Engineering and an M.S. in Chemical and Biochemical Engineering from Rutgers University and a B.S. in Chemical Engineering from Jadavpur University.
| 16 |
Anil Kapur, Chief Commercial Officer
Mr. Kapur joined Actinium in February 2018 from Bristol-Myers Squibb, where he was the Vice President, Head of Early Assets, Biomarkers & External Innovation within the Worldwide Oncology Commercialization organization and helped advance the company’s leading Immuno-Oncology portfolio. Prior to this position, he was the Vice President & Global Head, Oncology Commercial Portfolio & Product Strategy at Baxalta and a member of the Oncology Leadership Team. In this role, Mr. Kapur also led the Joint Strategic Committees responsible for advancing the early Immuno-Oncology partnerships with Symphogen and the allogeneic CAR-T partnership with Precision Bio-Sciences.
Mr. Kapur built a distinguished career spanning 15 years at Johnson & Johnson where in his last role, he served as the Vice President, Commercial Leader for the Hematology Franchise with responsibility for the development and execution of global commercial strategy and launch plans for all Hematology in-market, late-stage development, and early pipeline assets. He is credited with significantly shaping the clinical development plans and successful launch and growth of multiple Oncology blockbuster products including IMBRUVICA®, DARZALEX®, and VELCADE®.
At J&J, he led the IMBRUVICA® Joint Commercial Committee (JCC), established between J&J and Pharmacyclics, and built and led the global team that launched DARZALEX®, the first biologic for Multiple Myeloma. Anil also held leadership roles of increasing complexity and responsibility in US Marketing, US Regional Sales, and within the Asia-Pacific Regional Oncology organization covering 14 markets including Japan, China, Australia and Korea.
Mr. Kapur has an MBA from the Fuqua School of Business at Duke University, a MS in Industrial Engineering from Louisiana Tech University, and a Bachelor of Engineering from the Birla Institute of Technology, India.
Dale L. Ludwig, Ph.D., Chief Scientific Officer
Dr. Ludwig joined Actinium in January 2018. Dr. Ludwig has worked for 20 years in oncology antibody drug discovery and development at Eli Lilly and Company and at ImClone Systems, Inc., until its acquisition by Eli Lilly where he supported the development and successful launch of several biologic oncology drugs including Erbitux®, CyramzaTM, Portrazza®, and LartruvoTM as well as the clinical advancement of 10 additional therapeutic antibodies. Most recently, Dr. Ludwig served Chief Scientific Officer/Vice President of Oncology Discovery Research - Biologics Technology. In this role he was responsible for directing antibody discovery and development for oncology biologics and contributed to key strategic and project advancement efforts. Dr. Ludwig was a member of the Oncology Research Senior Leadership Team and directed the empowered antibody drug discovery programs that included collaborations with Immunogen and Zymeworks.
Prior to the acquisition of Imclone by Eli Lilly and Company, Dr. Ludwig served as Head of Molecular & Cellular Engineering at IMClone Systems Incorporated. In this capacity, Dr. Ludwig served as core team leader for several IND filings and phase 1 advancements for novel antibodies. In addition, he directed and oversaw the full spectrum of drug development including antibody discovery, screening, selection, engineering, optimization, cloning and expression. He was also tasked with establishing meaningful preclinical collaborations with key academic investigators and industry leaders. Post-acquisition he was the research representative to the ImClone-Lilly Transition Team.
Before his work in the biotechnology industry, Dr. Ludwig trained as a postdoctoral associate in the DNA Damage and Repair Group of the Los Alamos National Laboratory and as a postdoctoral fellow in the Department of Molecular Genetics, Biochemistry and Microbiology at the University of Cincinnati College of Medicine. Dr. Ludwig has a B.S. in biology with a concentration in microbiology from James Madison University and received his Ph.D. in Microbiology from East Carolina University.
| 17 |
Jeffrey W. Chell, MD, Director
Dr. Chell has been a director of the Company since April 2018. Dr. Chell is also a member of our Audit Committee and Compensation Committee. He has been the Chief Executive Officer Emeritus of the National Marrow Donor Program (NMDP) since 2017 having served as its CEO since 2000. Dr. Chell has led the NMDP through transformational growth as its Be The Match Registry tripled to more than 12 million donors, the number of transplants facilitated has grown five fold to over 6,400 annually, and revenue more than tripled to nearly $400 million per year. He is also the co-founder and has served as Executive Director of the Center For International Blood & Marrow Transplant Research since 2004, a leading research program in the field contributing over 70 research publications per year in peer-reviewed journals. Dr. Chell also currently serves as chair of CLR Insurance, a captive insurance company domiciled in the Cayman Islands. From 2014 to 2016, Dr. Chell served as co-chair of Bone Marrow Donors Worldwide (BMDW) during its IT transformation project, improving revenues and reducing costs.
Prior to joining the NMDP, he served as President, Allina Medical Clinics, a 450 physician multi-specialty medical group from 1994 to 1999. Prior to that he practiced Internal Medicine in Minneapolis and in the U.S. Air Force Medical Corps.
Dr. Chell received his M.D. from the University of Minnesota and his training in Internal Medicine at the University of Wisconsin, Madison. Dr. Chell is a diplomate of the American Board of Internal Medicine, a member of the American Society of Hematology and a member of the American Society of Blood and Marrow Transplantation.
He has received multiple honors including the 2018 Public Service award of the American Society For Blood and Marrow Transplantation, 2017 Most Admired CEO by the Minneapolis/St. Paul Business Journal, 2010 Healthcare Executive of the Year by the Minneapolis/St, Paul Business Journal, and the 2017 Bone Marrow Foundation Service Award.
That Dr. Chell has over 25 years of leadership and executive experience in healthcare, that he has significant knowledge in the blood and marrow transplantation field and that he has experience conducting business in the health sector, led us to conclude that Dr. Chell should serve as a director.
David Nicholson, PhD, Director
David Nicholson has been a Director of the Company since 2008. Dr. Nicholson is also a member of our Compensation Committee and Corporate Governance Committee. In August 2014, Dr. Nicholson joined Actavis plc and Forest Laboratories, Inc. as Senior Vice President, Actavis Global Brands R&D. From March 2012 to August 2014, Dr. Nicholson was on the Executive Committee of Bayer CropScience as Head of Research & Development responsible for the integration of the company’s R&D activities into one global organization. Dr. Nicholson graduated in pharmacology, earning his B.Sc. from the University of Manchester (1975) and his Ph.D. from the University of Wales (1980). Between 1978 and 1988, Dr. Nicholson worked in the pharmaceutical industry for the British company Beecham-Wülfing in Gronau, Germany. The main emphasis of his activities as group leader in a multidisciplinary project group was the development of cardiovascular drugs.
From 1988-2007, Dr. Nicholson held various positions of increasing seniority in the UK, the Netherlands and the USA with Organon, a Business Unit of Akzo Nobel. Ultimately, he became Executive Vice President, Research & Development, and member of the Organon Executive Management Committee. He implemented change programs, leading to maximizing effectiveness in research & development, ensuring customer focus and the establishment of a competitive pipeline of innovative drugs. In 2007, Dr. Nicholson transferred to Schering-Plough, Kenilworth, New Jersey as Senior Vice President, responsible for Global Project Management and Drug Safety. From 2009 to December 2011, he was Vice President Licensing and Knowledge Management at Merck in Rahway, New Jersey, reporting to the President of Merck R&D. As an integration team member, Dr. Nicholson played a role in the strategic mergers of Organon BioSciences, the human and animal health business of Dutch chemical giant Akzo-Nobel, and Schering-Plough in 2007 as well as of Schering-Plough and Merck in 2009.
That Dr. Nicholson brings over 25 years of pharmaceutical experience to our Board, having served in various pharmaceutical research and development executive-level positions over the course of his career, and that Dr. Nicholson has developed significant management and leadership skills relating to the pharmaceutical industry. and is well accustomed to interfacing with investors, analysts, auditors, outside advisors and governmental officials, led us to conclude that Dr. Nicholson should serve as a director.
| 18 |
Ajit S. Shetty, PhD, Director
Dr. Shetty has been a Director of the Company since March, 2017. Dr. Shetty is also a member of our, Audit Committee, Compensation Committee, and Chairman of our Corporate Governance Committee. Dr. Shetty joined Janssen Pharmaceutica, Inc. in 1976 ultimately rising to the position of President in 1986 where he led the establishment of Janssen’s business in the U.S. From 1999 to 2008 he was Managing Director of Janssen Pharmaceutica, during this time the Janssen Group of companies’ global sales grew from $1 billion to $8 billion, and from 2004 until 2012 he was Chairman of the Board of Directors. In Dr. Shetty’s most recent role at Johnson & Johnson he was head of Enterprise Supply Chain, where he reported to the CEO and was responsible for the transformation and optimization of Johnson & Johnson’s supply chain. Dr. Shetty earned a Ph.D. in Metallurgy and B.A. Natural Sciences from Trinity College, Cambridge University and a Master of Business Administration from Carnegie Mellon University. Dr. Shetty has served as a member of Agile Therapeutics, Inc.’s board of directors since February 2016. In 2007, Dr. Shetty was bestowed the title of Baron by King Albert II of Belgium for his exceptional merits. He is a member of the Board of Trustees of Carnegie Mellon University, serves on the Board of Governors for GS1 (Global Standards) in Belgium and formerly served on the Corporate Advisory Board of the John Hopkins Carey Business School. In 2016, Dr. Shetty was named as Chairperson of the Vlaams Instituut voor Biotechnologie (VIB), a Belgium based life sciences research institute focused on translating scientific results into pharmaceutical, agricultural and industrial applications. In addition, he was elected Manager of the Year in 2004 in Flanders and received a Life-Time Achievement Award in India in 2010. We believe Dr. Shetty’s qualifications to sit on our Board include his extensive pharmaceutical experience leading commercial and supply chain operations and his significant education background.
That Dr. Shetty has 37 years of leadership and executive experience in the pharmaceutical industry, that he has significant supply chain knowledge and that he has experience conducting business in the U.S. and Europe, led us to conclude that Dr. Shetty should serve as a director.
Richard I. Steinhart, MBA, Director
Mr. Steinhart has served as our Director and Chairman of the Audit Committee since November 2013. Mr. Steinhart is also a member of our Corporate Governance Committee. Since October 2017 Mr. Steinhart has been the Chief Financial Officer of BioXcel Therapeutics, Inc. Since March 2014, Mr. Steinhart has been a Member of the Board of Directors of Atossa Genetics, Inc. where he is Chairman of the Audit Committee and a member of the Compensation Committee. Form October 2015 to April 2017, Mr. Steinhart was Vice President and CFO at Remedy Pharmaceuticals, a privately-held, clinical stage pharmaceutical company. From January 2014 through September 2015 Mr. Steinhart had been a financial and strategic consultant to the biotechnology and medical device industries. From April 2006 through December 2013, Mr. Steinhart was employed by MELA Sciences, Inc., as their Vice President, Finance and Chief Financial Officer, Treasurer and Secretary. In April 2012, Mr. Steinhart received a promotion to Sr. Vice President, Finance and Chief Financial Officer. From May 1992 until joining MELA Sciences, Mr. Steinhart was a Managing Director of Forest Street Capital/SAE Ventures, a boutique investment banking, venture capital, and management consulting firm focused on healthcare and technology companies. Prior to Forest Street Capital/SAE Ventures, he was Vice President and Chief Financial Officer of Emisphere Technologies, Inc. Mr. Steinhart’s other experience includes seven years at CW Group, Inc., a venture capital firm focused on medical technology and biopharmaceutical companies, where he was a General Partner and Chief Financial Officer. Mr. Steinhart began his career at Price Waterhouse, now known as PricewaterhouseCoopers. He holds BBA and MBA degrees from Pace University and is a Certified Public Accountant (inactive).
That Mr. Steinhart brings nearly 30 years of financial experience to our Board, having served in various financial executive-level positions over the course of his career, and that Mr. Steinhart is a certified public accountant led us to conclude that Mr. Steinhart should serve as a director and chair the audit committee.
| 19 |
EXECUTIVE COMPENSATION
The following discussion provides compensation information pursuant to SEC rules and may contain statements regarding future individual and Company performance targets and goals. These targets and goals are disclosed in the limited context of the Company’s compensation programs and should not be understood to be statements of management’s expectations or estimates of results or other guidance. We specifically caution stockholders not to apply these statements to other contexts.
Chief Executive Officer’s Compensation
In August 2018, we amended and restated Mr. Seth’s, our Chief Executive Officer, August 6, 2015 Executive Chairman Agreement (the “Prior Agreement”), as amended. This new agreement (the “Agreement”) sets forth the terms related to his position as Chief Executive Officer and Chairman of the Board of the Company while retaining and adapting material provisions of the Prior Agreement to that of his role of Chief Executive Officer. Mr. Seth is currently paid an annual salary of $545,000. The Board reviews the amount of his base salary and performance bonus, and determines the appropriate adjustments to each component of his compensation each calendar year, and he may be entitled to a cash bonus in an amount to be determined by the board with a target of 50% of the base salary.
The Chairman and CEO shall also be awarded stock option and/or restricted stock grants at our Board’s discretion. Mr. Seth’s agreement includes severance benefits, including in the event of a change of control of the Company, and to provide for immediate vesting of options in accordance with our Amended and Restated 2013 Stock Plan. The term of the agreement is until February 21, 2021.
If the Company terminates the consulting arrangement other than for cause or if Mr. Seth resigns for good reason, Mr. Seth shall be entitled to the following:
| (i) | a single lump sum payment equal to twenty-four (24) months of Mr. Seth’s compensation (at the rate in effect as of the date of termination); |
| (ii) | continued health benefits for the 24-month period beginning on the date of termination; and |
| (iii) | All outstanding equity awards granted to Mr. Seth under the Company’s equity compensation plans shall become immediately vested and exercisable (as applicable) as of the date of such termination and the performance goals with respect to such outstanding performance awards, if any, will deemed satisfied at “target”. |
If the Company terminates Mr. Seth’s consulting arrangement other than for cause or if Mr. Seth resigns for good reason, in any case during the 12-month period beginning on the date of a change in control, Mr. Seth shall be entitled to the following:
| (i) | a single lump sum payment equal to thirty (30) months of Mr. Seth’s compensation (at the rate in effect as of the date of termination); |
| (ii) | continued health benefits for the 30-month period beginning on the date of termination; and |
| (iii) | All outstanding equity awards granted to Mr. Seth under the Company’s equity compensation plans shall become immediately vested and exercisable (as applicable) as of the date of such termination and the performance goals with respect to such outstanding performance awards, if any, will deemed satisfied at “target”. |
Mr. Seth is eligible to receive all standard benefits that Company employees are eligible to receive, including 20 vacation days and 5 sick days each year. The Company will provide Mr. Seth with standard business reimbursements (including mileage, supplies, long distance calls), subject to Company policies and procedures and with appropriate receipts. In addition, he will receive any other statutory benefits required by law.
The Company also entered in a standard Company indemnification agreement with Mr. Seth where the Company agreed to indemnify him in certain situations for his role as a Company officer.
Principal Financial Officer Compensation
In August 2018, we amended and restated Mr. O’Loughlin’s, our Principal Financial Officer, September 17, 2015 Employment Agreement (the “Prior Agreement”), as amended. This new agreement (the “Employment Agreement”) sets forth the terms related to his position as Principal Financial Officer of the Company while retaining and adapting material provisions of the Prior Agreement to that of his role of Principal Financial Officer.
| 20 |
Mr. O’Loughlin’s employment with the Company is on an “at will” basis, meaning that either Mr. O’Loughlin or the Company may terminate his employment at any time for any reason or no reason, without further obligation or liability, except as provided in his Employment Agreement. Pursuant to the Employment Agreement, Mr. O’Loughlin is entitled to the following compensation and benefits:
Salary and Bonus
The Board shall review the amount of his base salary and performance bonus, and shall determine the appropriate adjustments to each component of his compensation each calendar year. Mr. O’Loughlin’s is entitled to participate in an executive bonus program pursuant to which the Board may award him bonuses, based upon the achievement of written individual and corporate objectives such as the Board shall determine. Upon the attainment of such performance objectives, in addition to base salary, he shall be entitled to a cash bonus in an amount to be determined by the board with a target of 30% of the base salary. Mr. O’Loughlin’s current annual base salary is $285,000.
Options
From time to time the Board may grant him options or restricted stock to purchase common shares of the Company.
Non-Solicitation
Mr. O’Loughlin agreed that during the term of his employment with the Company, and for a period of 24 months following the cessation of employment with the Company for any reason or no reason, he shall not directly or indirectly solicit, induce, recruit or encourage any of our employees or consultants to terminate their relationship with us, or attempt any of the foregoing. For a period of 24 months following cessation of employment with us for any reason or no reason, he shall not attempt to negatively influence any of our clients or customers from purchasing our products or services.
Benefits
Mr. O’Loughlin is eligible to receive all standard benefits that Company employees are eligible to receive, including 20 vacation days and 5 sick days each year.
| Indemnification |
The Company also entered in a standard Company indemnification agreement with Mr. OLoughlin, where the Company agreed to indemnify him in certain situations for his role as a Company officer.
Chief Medical Officer Compensation
In December 2016, the Company and Dr. Mark S. Berger entered into an agreement (the “Berger Employment Agreement”), to employ Dr. Berger as our Chief Medical Officer. Dr. Berger’s employment with the Company is on an “at will” basis, meaning that either Dr,. Berger or the Company may terminate his employment at any time for any reason or no reason, without further obligation or liability, except as provided in his Employment Agreement.
Pursuant to the Berger Employment Agreement, Dr. Berger is entitled to the following compensation and benefits:
Dr. Berger’s current annual base salary is $400,000 per year. Dr. Berger may be entitled to a cash bonus in an amount to be determined by the Board with a target of 30% of the base salary.
From time to time the Board may grant him options or restricted stock to purchase common shares of the Company.
Dr. Berger is also eligible to participate in the Company’s benefit plans that are generally provided for executive employees.
Non-Competition. During the term and for a period of two years thereafter, Dr. Berger shall not, either directly or indirectly, engage (as principal, partner, employee, consultant, owner, independent contractor, advisor or otherwise, with or without compensation) in any business that directly or indirectly is developing, or plans to develop, radioimmunotherapies for cancer or any therapy related to bone marrow transplant (the “Competing Business”). Notwithstanding the foregoing, this does not prevent Dr. Berger from being engaged or employed with business that has a Competing Business as part of its business, so long as he is not engaged or involved in any way in the Competing Business at such business or enterprise.
| 21 |
Non-Solicitation. The employment agreement also contains a non-solicitation provision that provides that during the term of employment and for a period of 24 months following the cessation of employment with the company shall not directly or indirectly solicit, induce, recruit or encourage any of our employees or consultants to terminate their relationship with us, or attempt any of the foregoing, either for himself or any other person or entity.
The Company also entered in a standard Company indemnification agreement with Dr. Berger where the Company agreed to indemnify him in certain situations for his role as a Company officer.
Executive Vice-President, Head of Product Development, Manufacturing and Supply Chain Compensation
In May 2017 the Company and Dr. Nitya Ray entered into an Offer Letter (the “Employment Agreement”) pursuant to which Dr. Ray is the Company’s Executive Vice President, Head of Product Development, Manufacturing and Supply Chain. Dr. Ray’s employment with the Company is on an “at will” basis, meaning that either Dr. Ray or the Company may terminate his employment at any time for any reason or no reason, without further obligation or liability, except as provided in his Employment Agreement.
Pursuant to the Employment Agreement, Dr. Ray is entitled to the following compensation and benefits:
Dr. Ray’s current annual base salary is $330,850 per year, and Dr. Ray may be entitled to a cash bonus in an amount to be determined by the Board with a target of 30% of the base salary.
From time to time the Board may grant him options or restricted stock to purchase common shares of the Company.
Dr. Ray is also eligible to participate in our benefit plans that are generally provided for executive employees.
Non-Competition. During the term and for a period of three years thereafter, Dr. Ray shall not, either directly or indirectly, engage (as principal, partner, employee, consultant, owner, independent contractor, advisor or otherwise, with or without compensation) in any business that directly or indirectly is developing, or plans to develop, radioimmunotherapies for cancer or any therapy related to bone marrow transplant (the “Competing Business”). Notwithstanding the foregoing, this does not prevent Dr. Ray from being engaged or employed with business that has a Competing Business as part of its business, so long as he is not engaged or involved in any way in the Competing Business at such business or enterprise.
Non-Solicitation. The Employment Agreement also contains a non-solicitation provision that provides that during the term of employment and for a period of 24 months following the cessation of employment with the company shall not directly or indirectly solicit, induce, recruit or encourage any of the Company’s employees or consultants to terminate their relationship with the Company, or attempt any of the foregoing, either for himself or any other person or entity.
The Company also entered in a standard Company indemnification agreement with Dr. Ray where the Company agreed to indemnify him in certain situations for his role as a Company officer.
| 22 |
Chief Scientific Officer
The Company and Dr. Dale Ludwig, effective January 2018, entered into an Offer Letter (the “Employment Agreement”) pursuant to which Dr. Ludwig is the Company’s Chief Scientific Officer. Dr. Ludwig’s employment with the Company is on an “at will” basis, meaning that either Dr. Ludwig or the Company may terminate his employment at any time for any reason or no reason, without further obligation or liability, except as provided in his Employment Agreement. Pursuant to the Employment Agreement. Dr. Ludwig is entitled to the following compensation and benefits:
| ● | Dr. Ludwig’s current annual base salary is $325,000 per year, and Dr. Ludwig may be entitled to a cash bonus in an amount to be determined by the Board with a target of 30% of the base salary. |
| ● | The Board granted to Dr. Ludwig an option to purchase 200,000 common shares of the Company at an exercise price equal to the closing price of the Company’s common stock on January 8, 2018. |
| ● | Vesting Schedule. Twenty-eight percent (28%) of the initial options granted shall vest twelve months after the date of grant and two percent (2%) of the remainder shall vest each month thereafter until fully vested. The term of all options granted under this Agreement will be for 10 years from the date of grant, subject to Dr. Ludwig’ continuing service with the Company. |
| ● | Dr. Ludwig is also eligible to participate in the Company’s benefit plans that are generally provided for executive employees. |
| ● | Non-Competition. During the term and for a period of three (3) years thereafter, he shall not, either directly or indirectly, engage (as principal, partner, employee, consultant, owner, independent contractor, advisor or otherwise, with or without compensation) in any business that directly or indirectly competes with the Company (the “Competing Business”). Notwithstanding the foregoing, this does not prevent Dr. Ludwig from being engaged or employed with a business that has a Competing Business as part of its business, so long as he is not engaged or involved in any way in the Competing Business at such business or enterprise. |
Non-Solicitation. The employment agreement also contains a non-solicitation provision that provides that during the term of employment and for a period of 24 months following the cessation of employment with the company Dr. Ludwig shall not directly or indirectly solicit, induce, recruit or encourage any of the Company’s employees or consultants to terminate their relationship with the Company, or attempt any of the foregoing, either for himself or any other person or entity.
| ● | Indemnification. The Company also entered in a standard Company indemnification agreement with Mr. Ludwig where the Company agreed to indemnify him in certain situations for his role as a Company officer. |
Chief Commercial Officer
In January 2018 the Company and Anil Kapur entered into an Offer Letter (the “Employment Agreement”) pursuant to which Mr. Kapur is the Company’s Chief Commercial Officer. Mr. Kapur’s employment with the Company is on an “at will” basis, meaning that either Dr. Kapur or the Company may terminate his employment at any time for any reason or no reason, without further obligation or liability, except as provided in his Employment Agreement. Pursuant to the Employment Agreement. Pursuant to the Employment Agreement, Mr. Kapur is entitled to the following compensation and benefits:
| ● | Mr. Kapur’s current annual base salary is $325,000 per year, and he may be entitled to a cash bonus in an amount to be determined by the board with a target of 35% of the base salary. |
| ● | The board granted to Mr. Kapur an option to purchase 475,000 common shares of the Company at an exercise price equal to the closing price of the Company’s common stock on February 6, 2018. |
| ● | Vesting Schedule. Twenty-eight percent (28%) of the initial options granted shall vest twelve months after the date of grant and two percent (2%) of the remainder shall vest each month thereafter until fully vested. The term of all options granted under this Agreement will be for 10 years from the date of grant, subject to Mr. Kapur’s continuing service with the Company. |
| ● | Mr. Kapur is also eligible to participate in the Company’s benefit plans that are generally provided for executive employees. |
| ● | Non-Competition. During the term and for a period of three (3) years thereafter, Mr. Kapur shall not, either directly or indirectly, engage (as principal, partner, employee, consultant, owner, independent contractor, advisor or otherwise, with or without compensation) in any business that directly or indirectly is developing, or plans to develop, radioimmunotherapies for cancer or any therapy related to bone marrow transplant (the “Competing Business”). Notwithstanding the foregoing, this does not prevent him from being engaged or employed with a business that has a Competing Business as part of its business, so long as he is not engaged or involved in any way in the Competing Business at such business or enterprise. |
Non-Solicitation. The employment agreement also contains a non-solicitation provision that, among other things, provides that during the term of employment and for a period of 24 months following the cessation of employment with the Company he shall not directly or indirectly solicit, induce, recruit or encourage any of the Company’s employees or consultants to terminate their relationship with the Company, or attempt any of the foregoing, either for himself or any other person or entity.
| ● | Indemnification. The Company also entered in a standard Company indemnification agreement with Mr. Kapur where the Company agreed to indemnify him in certain situations for his role as a Company officer. |
| 23 |
Summary Compensation Table
The following table provides information regarding the compensation earned during the fiscal years ended December 31, 2017 and 2016 for our named executive officers.
| Name/Position | Year | Salary | Bonus (1) | Option Awards | All Other Compensation | Total | ||||||||||||||||
| Sandesh Seth (2) | 2017 | $ | 306,250 | $ | - | $ | - | $ | - | $ | 306,250 | |||||||||||
| Kaushik J. Dave, | 2017 | $ | 577,942 | $ | 110,000 | $ | 244,766 | $ | - | $ | 932,708 | |||||||||||
| Former CEO (3) | 2016 | $ | 405,000 | $ | 105,000 | $ | 571,150 | $ | 98,593 | $ | 1,179,743 | |||||||||||
| Mark Berger | 2017 | $ | 343,750 | $ | - | $ | 234,695 | $ | - | $ | 578,445 | |||||||||||
| Dragan Cicic, | 2017 | $ | 389,125 | $ | 45,000 | $ | 73,430 | $ | - | $ | 507,555 | |||||||||||
| Former COO (4) | 2016 | $ | 280,500 | $ | 40,000 | $ | 71,394 | $ | - | $ | 391,894 | |||||||||||
| Nitya Ray | 2017 | $ | 177,273 | $ | - | $ | 198,896 | $ | - | $ | 376,169 | |||||||||||
| Steve O’Loughlin | 2017 | $ | 235,152 | $ | 50,000 | $ | 97,907 | $ | - | $ | 383,059 | |||||||||||
| 2016 | $ | 192,610 | $ | 10,000 | $ | 71,394 | $ | - | $ | 274,004 | ||||||||||||
(1) The bonus disclosed in this column relates to performance in the prior year, but was contingent upon board approval, and was paid in the year disclosed.
(2) Mr. Seth was appointed Chief Executive Officer on June 5, 2017. Prior to this, Mr. Seth was Executive Chairman and was paid an annual consulting fee.
(3) Dr. Dave resigned from the company on May 12, 2017. His 2017 salary includes a severance of $410,000.
(4) Dr. Cicic resigned from the company on May 12, 2017. His 2017 salary includes a severance of $283,000.
As an “emerging growth company” we are not required to provide information relating to the ratio of total compensation of our Chief Executive Officer to the median of the annual total compensation of all of our employees, as required by the Investor Protection and Securities Reform Act of 2010, which is part of the Dodd-Frank Wall Street Reform and Consumer Protection Act.
| 24 |
Outstanding Equity Awards at Fiscal Year-End Table
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END - 2017
The following table sets forth all unexercised options and unvested restricted stock that have been awarded to our named executives by the Company and were outstanding as of December 31, 2017.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||
| Name (a) | Number of Securities Underlying Unexercised Options (#) (Exercisable) (b) | Number of Securities Underlying Unexercised Options (#) (Unexercisable) (c) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) (d) | Option Exercise Price ($) (e) | Option Expiration Date (f) | Number of Shares or Units of Stock That Have Not Vested (#) (g) | Market Value of Shares or Units of Stock That Have Not Vested ($) (h) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) (j) | |||||||||||||||||||||||||
| Sandesh Seth | 24,975 | - | - | 1.50 | 8/30/2022 | - | - | - | - | |||||||||||||||||||||||||
| 24,975 | - | - | 1.50 | 12/19/2022 | - | - | - | - | ||||||||||||||||||||||||||
| 218,400 | 61,600 | - | 6.13 | 9/23/2024 | - | - | - | - | ||||||||||||||||||||||||||
| 102,000 | 48,000 | - | 3.58 | 2/15/2025 | - | - | - | - | ||||||||||||||||||||||||||
| 200,000 | 300,000 | - | 1.99 | 4/15/2026 | - | - | - | - | ||||||||||||||||||||||||||
| 217,000 | 533,000 | - | 1.39 | 3/14/2027 | - | - | - | - | ||||||||||||||||||||||||||
| Mark Berger | - | 325,000 | - | 1.04 | 1/17/2027 | - | - | - | - | |||||||||||||||||||||||||
| Nitya Ray | - | 250,000 | - | 1.15 | 6/13/2027 | - | - | - | - | |||||||||||||||||||||||||
| Steve O’Loughlin | 56,000 | 44,000 | - | 1.79 | 9/28/2025 | - | - | - | - | |||||||||||||||||||||||||
| 20,000 | 30,000 | - | 1.99 | 4/15/2026 | - | - | - | - | ||||||||||||||||||||||||||
| 38,500 | 61,500 | - | 1.39 | 3/14/2027 | - | - | - | - | ||||||||||||||||||||||||||
Pension Benefits
At the present time, we do not sponsor a qualified or non-qualified defined benefit. Our Compensation Committee may elect to adopt qualified or non-qualified benefit plans in the future if it determines that doing so is in our best interest.
| 25 |
Potential Payments Under Severance/Change in Control Arrangements
The table below sets forth potential payments payable to our Chief Executive Officer in the event of a termination of employment or consulting arrangement, as applicable, under various circumstances. For purposes of calculating the potential payments set forth in the table below, we have assumed that (i) the date of termination was September 28, 2018 and (ii) the stock price was $0.7415, which was the closing market price of our common stock on September 28, 2018, the last business day of the third quarter.
| Name | Termination of Employment
Other Than for Cause or Resignation for Good Reason (Not in Connection with a Change in Control). ($) | Termination Following a Change
in Control without Cause or Executive Resigns with Good Reason ($) | ||||||
| Sandesh Seth | ||||||||
| Cash Payment | $ | 1,650,000 | $ | 2,062,500 | ||||
| Total Cash and Benefits | $ | 1,650,000 | $ | 2,062,500 | ||||
The term “change of control” means:
| (i) | the direct or indirect sale, transfer, conveyance or other disposition (other than by way of merger or consolidation), in one or a series of related transactions, of all or substantially all of the properties or assets of the Company and its subsidiaries, taken as a whole, to any “Person” (as that term is used in Section 13(d)(3) of the Exchange Act) that is not an Affiliate; |
| (ii) | the “Incumbent Directors” (meaning those individuals who, on date the Plan was adopted by the Board (the “Effective Date”), constitute the Board, provided that any individual becoming a Director subsequent to the Effective Date whose election or nomination for election to the Board was approved by a vote of at least two-thirds of the Incumbent Directors then on the Board (either by a specific vote or by approval of the proxy statement of the Company in which such person is named as a nominee for Director without objection to such nomination) shall be an Incumbent Director, and further provided that no individual initially elected or nominated as a director of the Company as a result of an actual or threatened election contest with respect to Directors or as a result of any other actual or threatened solicitation of proxies by or on behalf of any person other than the Board shall be an Incumbent Director) cease for any reason to constitute at least a majority of the Board; |
| (iii) | the date which is 10 business days prior to the consummation of a complete liquidation or dissolution of the Company; |
| (iv) | the acquisition by any Person of “Beneficial Ownership” (within the meaning of Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating the Beneficial Ownership of any particular Person, such Person shall be deemed to have beneficial ownership of all securities that such Person has the right to acquire by conversion or exercise of other securities, whether such right is currently exercisable or is exercisable only after the passage of time) of 50% or more (on a fully diluted basis) of either (A) the then outstanding shares of Common Stock of the Company, taking into account as outstanding for this purpose such Common Stock issuable upon the exercise of options or warrants, the conversion of convertible stock or debt, and the exercise of any similar right to acquire such Common Stock (the “Outstanding Company Common Stock”) or (B) the combined voting power of the then outstanding voting securities of the Company entitled to vote generally in the election of directors (the “Outstanding Company Voting Securities”); provided, however, that for purposes of this Plan, the following acquisitions shall not constitute a Change of Control: (I) any acquisition by the Company or any Affiliate, (II) any acquisition by any employee benefit plan sponsored or maintained by the Company or any Affiliate, (III) any acquisition which complies with clauses, (A), (B) and (C) of subsection (v) of this definition, or (IV) in respect of an Award held by a particular Participant, any acquisition by the Participant or any group of persons including the Participant (or any entity controlled by the Participant or any group of persons including the Participant); or |
| 26 |
| (v) | the consummation of a reorganization, merger, consolidation, statutory share exchange or similar form of corporate transaction involving the Company that requires the approval of the Company’s shareholders, whether for such transaction or the issuance of securities in the transaction (a “Business Combination”), unless immediately following such Business Combination: (A) more than 50% of the total voting power of (I) the entity resulting from such Business Combination (the “Surviving Company”), or (II) if applicable, the ultimate parent entity that directly or indirectly has beneficial ownership of sufficient voting securities eligible to elect a majority of the members of the board of directors (or the analogous governing body) of the Surviving Company (the “Parent Company”), is represented by the Outstanding Company Voting Securities that were outstanding immediately prior to such Business Combination (or, if applicable, is represented by shares into which the Outstanding Company Voting Securities were converted pursuant to such Business Combination), and such voting power among the holders thereof is in substantially the same proportion as the voting power of the Outstanding Company Voting Securities among the holders thereof immediately prior to the Business Combination; (B) no Person (other than any employee benefit plan sponsored or maintained by the Surviving Company or the Parent Company) is or becomes the Beneficial Owner, directly or indirectly, of 50% or more of the total voting power of the outstanding voting securities eligible to elect members of the board of directors of the Parent Company (or the analogous governing body) (or, if there is no Parent Company, the Surviving Company); and (C) at least a majority of the members of the board of directors (or the analogous governing body) of the Parent Company (or, if there is no Parent Company, the Surviving Company) following the consummation of the Business Combination were Board members at the time of the Board’s approval of the execution of the initial agreement providing for such Business Combination |
The cash component (as opposed to option accelerations) of any change of control payment would be structured as a one-time cash severance payment.
PRINCIPAL STOCKHOLDERS
The following table shows the beneficial ownership of our Common Stock as of October 23, 2018 held by (i) each person known to us to be the beneficial owner of more than five percent (5%) of any class of our shares; (ii) each director; (iii) each executive officer; and (iv) all directors and executive officers as a group.
Beneficial ownership is determined in accordance with the rules of the SEC, and generally includes voting power and/or investment power with respect to the securities held. Shares of Common Stock subject to options and warrants currently exercisable or which may become exercisable within 60 days of October 23, 2018, are deemed outstanding and beneficially owned by the person holding such options or warrants for purposes of computing the number of shares and percentage beneficially owned by such person, but are not deemed outstanding for purposes of computing the percentage beneficially owned by any other person. Except as indicated in the footnotes to this table, the persons or entities named have sole voting and investment power with respect to all shares of our Common Stock shown as beneficially owned by them.
Unless otherwise indicated, the principal address of each of the persons below is c/o Actinium Pharmaceuticals, Inc., 275 Madison Ave, 7th floor, New York, NY 10016.
| Executive Officers and Directors | Number of Shares of Common Stock and Preferred Stock Beneficially Owned | Percentage of Ownership(a) | ||||||
| Sandesh Seth | 1,611,147 | (1) | 1.4 | % | ||||
| Steve O’Loughlin | 230,500 | (2) | * | % | ||||
| Mark Berger, M.D. | 220,500 | (3) | * | % | ||||
| Nitya G. Ray, Ph.D. | 137,500 | (4) | * | % | ||||
| Anil Kapur | 40,000 | (5) | * | % | ||||
| Dale Ludwig, Ph.D. | - | (6) | * | % | ||||
| Jeffrey W. Chell, M.D. | 7,500 | (7) | * | % | ||||
| David Nicholson, Ph.D. | 219,900 | (8) | * | % | ||||
| Ajit S. Shetty, Ph.D. | 64,730 | (9) | * | % | ||||
| Richard I. Steinhart | 179,447 | (10) | * | % | ||||
| All Directors and Officers as a Group (10 persons) | 2,711,224 | 2.3 | % | |||||
| All other 5% holders | ||||||||
| Anson Funds Management LP, Anson Management GP LLC, Mr. Bruce R. Winson, Anson Advisors
Inc., Mr. Amin Nathoo and Mr. Moez Kassam 5950 Berkshire Lane, Suite 210 Dallas, Texas 75225 | 6,000,000 | 5.5 | % | |||||
| * | less than 1% |
| (a) | Based on 114,698,044 shares of Common Stock outstanding as of October 23, 2018 |
| 27 |
| (1) | Warrants to purchase an aggregate of 64,747 shares of Common Stock of the Company at an exercise price of $0.784 per share, exercisable on a cashless basis, warrants to purchase an aggregate of 99,617 of Common Stock of the Company at an exercise price of $0.784 per share, exercisable on a cashless basis issued to Amrosan, LLC, a partnership in which the majority member interest is owned by the family of Mr. Seth, and warrants to purchase 57,212 shares of Common Stock at an exercise price of $2.34 per share. Excludes warrants to purchase an aggregate of 375,556 shares of Common Stock of the Company at par value per share, exercisable on a cashless basis issued to Amrosan, LLC as the warrants are not exercisable upon less than 90 days’ notice. The holder may waive the 90-day exercise notice requirement by giving 65 days prior notice of such waiver. Excludes 353,023 warrants issued to Carnegie Hill Asset Partners and irrevocable trust linked to Mr. Seth’s family and 721,068 warrants issued to Bioche Asset Management, LLC, a partnership in which the majority member interest is owned by the family of Mr. Seth whose terms are the same as those issued to Amrosan LLC. On August 30, 2012 and December 12, 2012, Mr. Seth was granted options to purchase an aggregate of 49,950 shares of Common Stock at an exercise price of $1.50 per share. On September 13, 2014, Mr. Seth was granted an option to purchase 280,000 shares with an exercise price of $6.13 per share. On February 18, 2015, Mr. Seth was granted an option to purchase 150,000 shares with an exercise price of $3.58 per share. On April 15, 2016, Mr. Seth was granted an option to purchase 500,000 shares at an exercise price of $1.99 per share. On March 14, 2017, Mr. Seth was granted options to purchase an aggregate of 750,000 shares of Common Stock at an exercise price of $1.39 per share. On July 13, 2018, Mr. Seth was granted an option to purchase 1,000,000 shares at an exercise price of $0.7829 per share. All options are subject to vesting. Within 60 days of October 23, 2018, 1,260,950 options will have vested. Includes 133,333 shares of Common Stock, 13,125 March 2018 Series A Warrants and 39,375 March 2018 Series B Warrants. |
| (2) | On October 1, 2015, Mr. O’Loughlin was granted an option to purchase 100,000 shares with an exercise price of $1.79 per share. On April 14, 2016, Mr. O’Loughlin was granted an option to purchase 50,000 shares at an exercise price of $1.99 per share. On March 14, 2017, Mr. O’Loughlin was granted options to purchase an aggregate of 100,000 shares at an exercise price of $1.39 per share. On July 13, 2018, Mr. O’Loughlin was granted an option to purchase 265,000 shares at an exercise price of $0.7829 per share. All options are subject to vesting. Within 60 days of October 23, 2018, 195,000 options will have vested. Includes 25,500 shares of Common Stock, 2,500 March 2018 Series A Warrants and 7,500 March 2018 Series B Warrants. |
| (3) | On January 17, 2017, Dr. Berger was granted an option to purchase 325,000 shares with an exercise price of $1.04 per share. On July 13, 2018, Dr. Berger was granted an option to purchase 250,000 shares at an exercise price of $0.7829. All options are subject to vesting. Within 60 days of October 23, 2018, 187,500 shares will have vested. Includes 19,000 shares of Common Stock, 3,500 March 2018 Series A Warrants and 10,500 March 2018 Series B Warrants. |
| (4) | On June 15, 2017, Dr. Ray was granted an option to purchase 250,000 shares with an exercise price of $1.15 per share. On July 13, 2018, Dr. Ray was granted an option to purchase 75,000 shares with an exercise price of $0.7829 per share. All options are subject to vesting. Within 60 days of October 23, 2018, 107,500 shares will have vested. Includes 20,000 shares of Common Stock, 2,500 March 2018 Series A Warrants and 7,500 March 2018 Series B Warrants. |
| (5) | On February 6, 2018, Mr. Kapur was granted an option to purchase 475,000 shares of Common Stock at an exercise price of $0.64 per share. It is not exercisable with 60 days of October 23, 2018. Includes 40,000 shares of Common Stock. |
| (6) | On January 8, 2018, Dr. Ludwig was granted an option to purchase 200,000 shares of Common Stock at an exercise price of $0.72 per share. It is not exercisable within 60 days of October 23, 2018. |
| (7) | On April 27, 2018, Dr. Chell was granted an option to purchase 75,000 shares of Common Stock at an exercise price of $0.347 per share. On July 13, 2018, Dr. Chell was granted an option to purchase 75,000 shares at an exercise price of $0.7829 per share. Within 60 days of October 23, 2018, 7,500 shares will have vested. |
| (8) | On February 12, 2012, Dr. Nicholson was granted an option to purchase 49,950 shares of Common Stock at an exercise price of $0.784 per share and on August 12, 2012 and December 19, 2012, Dr. Nicholson was granted options to purchase an aggregate of 49,950 shares at an exercise price of $1.50 per share. On February 18, 2015, Dr. Nicholson was granted an option to purchase 25,000 shares with an exercise price of $3.58 per share. On April 15, 2016, Dr. Nicholson was granted an option to purchase 75,000 shares at an exercise price of $1.99 per share. On March 14, 2017, Dr. Nicholson was granted an option to purchase 75,000 shares at an exercise price of $1.39 per share. On July 13, 2018, Dr. Nicholson was granted an option to purchase 75,000 shares at an exercise price of $0.7829 per share. All options are subject to vesting. Within 60 days of October 23, 2018, 209,900 options will have vested. Includes 10,000 shares of Common Stock. |
| (9) | On March 28, 2017, Dr. Shetty was granted an option to purchase 75,000 shares of Common Stock with an exercise price of $1.58 per share. On July 13, 2018, Dr. Shetty was granted an option to purchase 75,000 shares at an exercise price of $0.7829 per share. Within 60 days of October 23, 2018, 42,000 shares will have vested. Includes 22,730 shares of Common Stock. |
| (10) | On December 16, 2013 Mr. Steinhart was granted an option to purchase 49,950 shares of Common Stock at an exercise price of $6.70 per share. On February 18, 2015, Mr. Steinhart was granted an option to purchase 25,000 shares at an exercise price of $3.58 per share. On April 15, 2016, Mr. Steinhart was granted an option to purchase 75,000 shares at an exercise price of $1.99 per share. On March 14, 2017, Mr. Steinhart was granted an option to purchase 75,000 shares at an exercise price of $1.39 per share. On July 13, 2018, Mr Steinhart was granted an option to purchase 75,000 shares at an exercise price of $0.7829 per share. All options are subject to vesting. Within 60 days of October 23, 2018, 162,947 options will have vested. Includes 9,500 shares of Common Stock, 1,750 March 2018 Series A Warrants and 5,250 March 2018 Series B Warrants. |
| 28 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The following sets forth a summary of transactions, or any currently proposed transaction, in which the Company was to be a participant and the amount involved exceeded or exceeds $120,000 and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation”). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
Transactions with Related Persons
On February 11, 2015, the Company completed a public offering of common shares and warrants, representing gross proceeds of approximately $20.0 million and a net amount of approximately $18.5 million after deducting the underwriting discount and the other offering expenses. Laidlaw & Company (UK) Ltd., of which Mr. Seth, Chairman and CEO was the former Head of Healthcare Investment Banking, acted as sole book-running manager for the offering. The offering was made pursuant to a shelf registration statement (File No. 333-194768) previously filed with and declared effective by the U.S. Securities and Exchange Commission.
In an agreement dated March 11, 2015 and effective August 11, 2014, Mr. Seth entered into a consulting agreement with the Company to serve as Executive Chairman of the Company. The agreement was amended and restated on August 6, 2015. This agreement was later amended and restated in August 2018, to take in account Mr. Seth’s position as Chairman and CEO of the Company. Mr. Seth is also entitled to participate in a Company bonus program, which shall be established by the Board pursuant to which the Board shall award bonuses to the consultant, based upon the achievement of written individual and corporate objectives such as the Board shall determine..
Non-Competition Agreements and Indemnity Agreements
Our executive officers have signed non-competition agreements, which provide that all inventions become the immediate property of our Company and require invention assignments. The agreements provide that the executive officers will hold proprietary information in the strictest confidence and not use the confidential information for any purpose not expressly authorized by us. Our executive officers and directors have also entered into indemnity agreements with us.
MATTERS TO BE CONSIDERED AT THE ANNUAL MEETING
PROPOSAL 1
ELECTION OF DIRECTORS
Nominee for the Board of Directors
The Company’s Certificate of Incorporation (the “Charter”) established a classified Board of Directors with three classes of directors. Currently there are four directors divided into three classes designated Class I, Class II, and Class III. The term of office for each Class I director expires at the 2020 Annual Meeting; the term of office for each Class II director expires at the 2018 annual meeting of shareholders; and the term of office for each Class III director expires at the 2019 annual meeting of shareholders. Pursuant to the Charter, the directors due for election at the 2018 Annual meeting are Jeffrey W. Chell and Sandesh Seth (Class II).
| 29 |
The Board of Directors proposes the election of the following individuals to serve on its Board of Directors for a term that continues pursuant to the director terms outlined below or until his successor is duly elected. The nominees are current board members Dr. Chell and Mr. Seth. In the event the nominee is unable or unwilling to serve as a director, the individual named as proxy on the proxy card will vote the shares that he represents for election of such other person or persons as the Board of Directors may recommend. The Board of Directors has no reason to believe that the nominee will be unable or unwilling to serve.
The following is information about the nominee, including biographical data for at least the last five years. Should the nominees become unavailable to accept nomination or election as a director, the individual named as proxy on the enclosed proxy card will vote the shares that he represents for the election of such other person as the Board of Directors may recommend.
The Board of Directors is responsible for supervision of the overall affairs of the company. Following the annual meeting, the Board of Directors will consist of four directors. The term of each director is set forth below or until their successors are duly elected:
| Director | Class | Term (from 2018 Annual Meeting, if elected) | ||
| David Nicholson | Class I | 2 years | ||
| Richard I Steinhart | Class I | 2 years | ||
| Jeffrey W. Chell | Class II | 3 years | ||
| Sandesh Seth | Class II | 3 years | ||
| Ajit S. Shetty | Class III | 1 years |
Directors elected at each annual meeting shall be elected for a 3-year term. The name of the nominee for our Board of Directors and information about him is set forth below. There are no family relationships between any of the executive officers and directors.
Jeffrey W. Chell, MD, Director
Dr. Chell has been a director of the Company since April 2018. Dr. Chell is also a member of our Audit Committee and Compensation Committee. He has been the Chief Executive Officer Emeritus of the National Marrow Donor Program (NMDP) since 2017, having served as its CEO since 2000. Dr. Chell has led the NMDP through transformational growth as its Be The Match Registry tripled to more than 12 million donors, the number of transplants facilitated has grown fivefold to over 6,400 annually, and revenue more than tripled to nearly $400 million per year. He is also the co-founder and has served as Executive Director of the Center For International Blood & Marrow Transplant Research since 2004, a leading research program in the field, contributing over 70 research publications per year in peer-reviewed journals. Dr. Chell also currently serves as chair of CLR Insurance, a captive insurance company domiciled in the Cayman Islands. From 2014 to 2016, Dr. Chell served as co-chair of Bone Marrow Donors Worldwide (BMDW) during its IT transformation project, improving revenues and reducing costs.
Prior to joining the NMDP, he served as President, Allina Medical Clinics, a 450 physician multi-specialty medical group from 1994 to 1999. Prior to that he practiced Internal Medicine in Minneapolis and in the U.S. Air Force Medical Corps.
Dr. Chell received his M.D. from the University of Minnesota and his training in Internal Medicine at the University of Wisconsin, Madison. Dr. Chell is a diplomate of the American Board of Internal Medicine, a member of the American Society of Hematology and a member of the American Society of Blood and Marrow Transplantation.
He has received multiple honors including the 2018 Public Service award of the American Society For Blood and Marrow Transplantation, 2017 Most Admired CEO by the Minneapolis/St. Paul Business Journal, 2010 Healthcare Executive of the Year by the Minneapolis/St, Paul Business Journal, and the 2017 Bone Marrow Foundation Service Award.
That Dr. Chell has over 25 years of leadership and executive experience in healthcare, that he has significant knowledge in the blood and marrow transplantation field and that he has experience conducting business in the health sector, led us to conclude that Dr. Chell should serve as a director.
| 30 |
Sandesh Seth, MS, MBA, Chairman and CEO
Mr. Sandesh Seth has been our Chief Executive Officer since June 2017. Mr. Seth has been a Director since March 2012, our Chairman of the Board since October 2013, and served as Executive Chairman from August 2014 to June 2017. Mr. Seth was affiliated with Laidlaw & Co. (UK) Ltd., a healthcare focused, investment banking and wealth management firm where he was Head of Healthcare Investment Banking. Mr. Seth is the Chairman of the Board of Relmada Therapeutics, Inc., a publicly listed, specialty pharmaceuticals company focused on pain therapeutics.
Mr. Seth has over 25 years of experience in investment banking (Cowen & Co.), equity research (Bear Stearns, Commonwealth Associates) and in the pharma industry (Pfizer, Warner-Lambert, SmithKline in strategic planning, business development and R&D project management). Mr. Seth has an MBA in Finance from New York University; an M.S. in the Pharmaceutical Sciences from the University of Oklahoma Health Center and a B.Sc. in Chemistry from Bombay University. He has published several scientific articles and was awarded the University Regents Award for Research Excellence at the University of Oklahoma. Mr. Seth was designated as Regulatory Affairs Certified (R.A.C.) by the Regulatory Affairs Professionals Society which signifies proficiency with U.S. FDA regulations.
That Mr. Seth has served in various business executive-level positions over the course of his career, has significant investment banking experience, has developed significant management and leadership skills and is well accustomed to interfacing with investors, analysts, auditors, C-level executives, and outside advisors, led us to conclude that Mr. Seth should serve as a director.
Vote Required
Directors are elected by a plurality of the votes cast in person or by proxy at the annual meeting and entitled to vote on the election of directors. “Plurality” means that the nominees receiving the greatest number of affirmative votes will be elected as directors, up to the number of directors to be chosen at the meeting. Broker non-votes will not affect the outcome of the election of directors because brokers do not have discretion to cast votes on this proposal without instruction from the beneficial owner of the shares.
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE “FOR” ELECTION OF
THE DIRECTOR NOMINEES
PROPOSAL 2
RATIFICATION OF THE APPOINTMENT OF MARCUM LLP
The audit committee has appointed Marcum LLP as our independent registered public accounting firm to audit the consolidated financial statements of Actinium Pharmaceuticals, Inc. and its subsidiaries for the fiscal year ending December 31, 2018. Representatives of Marcum LLP will be present at the annual meeting and will have an opportunity to make a statement or to respond to appropriate questions from stockholders. Although stockholder ratification of the appointment of our independent auditor is not required by our Bylaws or otherwise, we are submitting the selection of Marcum LLP to our stockholders for ratification to permit stockholders to participate in this important corporate decision. If not ratified, the audit committee will reconsider the selection, although the audit committee will not be required to select a different independent auditor for our company.
Vote Required
The ratification of the appointment of Marcum LLP as our independent registered public accounting firm will be approved if there is a quorum and the votes cast “FOR” the proposal exceeds those cast against the proposal.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” RATIFICATION OF MARCUM LLP AS THE INDEPENDENT REGISTERED ACCOUNTING FIRM OF ACTINIUM PHARMACEUTICALS, INC.
| 31 |
PROPOSAL 3
APPROVAL OF AMENDMENT OF THE 2013 AMENDED AND RESTATED STOCK PLAN, AS AMENDED, TO INCREASE THE NUMBER OF SHARES OF COMMON STOCK AUTHORIZED FOR ISSUANCE UNDER THE PLAN BY 5 MILLION SHARES FROM 17,750,000 TO 22,750,000 TO SUPPORT PLANNED HIRING EFFORTS AS THE COMPANY GROWS
Description of Proposed Amendment
On September 28, 2018, the Board unanimously approved an amendment (the “Plan Amendment”) to the Company’s 2013 Amended and Restated Stock Plan, as amended (the “Plan”), subject to stockholder approval, to increase the number of shares of Common Stock authorized for issuance under the Plan by 5 million shares from 17,750,000 to 22,750,000 to support planned hiring efforts as the company grows.
The full text of the proposed Plan Amendment is set out in Annex A to this Proxy Statement. The text of the proposed Plan Amendment is subject to modification to include such changes as the Board deems necessary and advisable to affect the increase in the number of shares of Common Stock reserved and available for issuance under the Plan. Stockholders are being asked to approve the Plan Amendment.
Vote Required and Recommendation
The approval of the Plan Amendment will be made upon the affirmative vote of the majority of shares cast on the proposal. Abstentions and broker non-votes will have no direct effect on the outcome of this proposal. If the proposal is not approved by the stockholders, the Plan Amendment will not be effective and the proposal will not be implemented.
Reasons for the Plan Amendment
Existing Plan
Our Plan is currently comprised of 17,750,000 shares of Common Stock.
The purpose of the Plan is to provide a means through which we may attract and retain key personnel and to provide a means whereby directors, officers, managers, employees, consultants and advisors (and prospective directors, officers, managers, employees, consultants and advisors) can acquire and maintain an equity interest in our Company, or be paid incentive compensation, which may (but need not) be measured by reference to the value of common shares, thereby strengthening their commitment to the welfare of our Company and aligning their interests with those of our stockholders.
Increase in Size of Plan
As of September 30, 2018, outstanding awards (consisting of options to purchase shares of common stock) issued under the Plan totaled 7,667,271 shares of common stock, leaving 9,481,065 shares available for issuance.
Our Board determined to increase the number of shares of common stock reserved and available for issuance under the Plan by 5 million shares because it believes that the current number is insufficient for the purposes of the Plan for future issuances. The market for quality personnel is competitive, and the ability to obtain and retain competent personnel is of great importance to our business operations.
Effects of the Plan Amendment
As a result of the Plan Amendment, there will be an increase in the total number of shares of common stock reserved for issuance under the Plan. This will provide us with the ability to grant more awards than are currently available under the Plan to eligible recipients including employees, directors, consultants and advisors. The issuance in the future of awards under the Plan consisting of full value awards and options to purchase shares of common stock may have the effect of diluting the earnings per share and book value per share, as well as the stock ownership and voting rights, of the holders of the currently outstanding shares of common stock. The effective increase in the number of authorized but unissued shares of common stock which may be issued as awards under the Plan may be construed as having an anti-takeover effect by permitting the issuance of shares to purchasers who might oppose a hostile takeover bid or oppose any efforts to amend or repeal certain provisions of our Certificate of Incorporation or Bylaws. Holders of our common stock have no preemptive or other subscription rights. There are no other material differences to the Plan as a result of the Plan Amendment.
| 32 |
Material Terms of the Plan
Purpose. The purposes of the 2013 Stock Plan, as amended, are to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to employees, directors and consultants and to promote the success of our business.
Administration. The Plan shall be administered by our Board or a committee, or a combination thereof, as determined by our Board. The Plan may be administered by different administrative bodies with respect to different classes of participants and, if permitted by the applicable laws, the board may authorize one or more officers to make awards under the Plan.
Powers of the Administrator. The specific duties delegated by our Board to the committee, the administrator shall have the authority, in its discretion:
to determine the fair market value of the common stock, provided that such determination shall be applied consistently with respect to participants under the Plan;
to select the employees, directors and consultants to whom Plan awards may from time to time be granted:
to determine whether and to what extent Plan awards are granted;
to determine the number of shares of common stock to be covered by each award granted;
to approve the form(s) of agreement(s) used under the Plan;
to determine the terms and conditions, not inconsistent with the terms of the Plan, of any award granted hereunder, which terms and conditions include but are not limited to the exercise or purchase price, the time or times when awards may be exercised (which may be based on performance criteria), any vesting acceleration or waiver of forfeiture restrictions, any pro-rata adjustment to vesting as a result of a Participant’s transitioning from full-to part-time services (or vice versa), and any restriction or limitation regarding any option, optioned stock, stock purchase right or restricted stock, based in each case on such factors as the Administrator, in its sole discretion, shall determine;
to adjust the vesting of an option held by an employee, director or consultant as a result of a change in the terms or conditions under which such person is providing services to us;
to construe and interpret the terms of the Plan and awards granted under the Plan, which constructions, interpretations and decisions shall be final and binding on all participants; and
in order to fulfill the purposes of the Plan and without amending the Plan, to modify grants of options or stock purchase rights to participants who are foreign nationals or employed outside of the United States in order to recognize differences in local law, tax policies or customs.
Eligibility. Nonstatutory Stock Options and Stock Purchase Rights may be granted to employees, directors and consultants. Incentive Stock Options may be granted only to employees, provided that employees of affiliates shall not be eligible to receive Incentive Stock Options. Each option shall be designated in the option agreement as either an Incentive Stock Option or a Nonstatutory Stock Option. To the extent that the aggregate fair market value of shares with respect to which options designated as Incentive Stock Options are exercisable for the first time by any optionee during any calendar year exceeds $100,000, such excess options shall be treated as Nonstatutory Stock Options.
Term of Plan. The Plan shall become effective upon its adoption by our Board. It shall continue in effect for a term of ten (10) years.
Term of Option. The term of each option shall be the term stated in the option agreement; provided that the term shall be no more than ten years from the date of grant thereof or such shorter term as may be provided in the option agreement and provided further that, in the case of an incentive stock option granted to a person who at the time of such grant is a holder of ten percent or more of our outstanding shares, the term of the option shall be five years from the date of grant thereof or such shorter term as may be provided in the option agreement.
| 33 |
Option Exercise Price and Consideration. The per share exercise price for the shares to be issued pursuant to exercise of an option shall be such price as is determined by the administrator and set forth in the option agreement, but shall be subject to the following:
In the case of an Incentive Stock Option
| (A) | granted to an employee who at the time of grant is a holder of ten percent or more of our outstanding shares, the per share exercise price shall be no less than 110% of the fair market value per share on the date of grant; or |
| (B) | granted to any other employee, the per share exercise price shall be no less than 100% of the fair market value per share on the date of grant. |
In the case of a Nonstatutory Stock Option, the per share exercise price shall be such price as determined by the administrator provided that for any Nonstatutory Stock Option granted on any date on which the common stock is a listed security to an eligible person who is, at the time of the grant of such option, a named executive of the Company, the per share exercise price shall be no less than 100% of the fair market value on the date of grant if such option is intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code.
Exercise of Option. Any option granted hereunder shall be exercisable at such times and under such conditions as determined by the administrator, consistent with the term of the Plan and reflected in the option agreement, including vesting requirements and/or performance criteria with respect to the Company and/or the optionee. The administrator shall have the discretion to determine whether and to what extent the vesting of options shall be tolled during any unpaid leave of absence; provided however that in the absence of such determination, vesting of options shall be tolled during any such leave (unless otherwise required by the Applicable Laws.
Until the issuance of the shares (as evidenced by the appropriate entry on the books of the Company or of a duly authorized transfer agent of the Company), no right to vote or receive dividends or any other rights as a stockholder shall exist with respect to the optioned stock, notwithstanding the exercise of the option.
Termination of Employment or Consulting Relationship. Except as otherwise set forth in the plan the administrator shall establish and set forth in the applicable option agreement the terms and conditions upon which an option shall remain exercisable, if at all, following termination of an optionee’s continuous service status, which provisions may be waived or modified by the administrator at any time in the Administrator’s sole discretion. Unless otherwise provided in the option agreement, to the extent that the optionee is not vested in the optioned stock on the date of termination of his or her continuous service status, or if the optionee (or other person entitled to exercise the option) does not exercise the option to the extent so entitled within the time specified in the option agreement or below (as applicable), the option shall terminate and the optioned stock underlying the unexercised portion of the option shall revert to the Plan. In no event may any option be exercised after the expiration of the option term as set forth in the option agreement.
The following provisions (1) shall apply to the extent an option agreement does not specify the terms and conditions upon which an option shall terminate upon termination of an optionee’s continuous service status, and (2) establish the minimum post-termination exercise periods that may be set forth in an option agreement:
Termination other than Upon Disability or Death. In the event of termination of an optionee’s continuous service status, such optionee may exercise an option for 30 days following such termination to the extent the optionee was vested in the optioned stock as of the date of such termination.
Disability of Optionee. In the event of termination of an optionee’s continuous service status as a result of his or her disability (including a disability within the meaning of Section 22(e)(3) of the Internal Revenue Code), such optionee may exercise an option at any time within twelve months following such termination to the extent the optionee was vested in the optioned stock as of the date of such termination.
Death of Optionee. In the event of the death of an optionee during the period of continuous service status since the date of grant of the option, or within thirty days following termination of optionee’s continuous service, the option may be exercised by optionee’s estate or by a person who acquired the right to exercise the option by bequest or inheritance at any time within twelve months following the date of death, but only to the extent the optionee was vested in the optioned stock as of the date of death or, if earlier, the date the optionee’s continuous service status terminated.
Buyout Provisions. The administrator may at any time offer to buy out for a payment in cash or shares an option previously granted under the Plan based on such terms and conditions as the administrator shall establish and communicate to the optionee at the time that such offer is made.
| 34 |
Stock Purchase Rights. When the administrator determines that it will offer stock purchase rights under the Plan, it shall advise the offeree in writing of the terms, conditions and restrictions related to the offer, including the number of shares that such person shall be entitled to purchase, the price to be paid, and the time within which such person must accept such offer. The offer to purchase shares subject to stock purchase rights shall be accepted by execution of a restricted stock purchase agreement in the form determined by the Administrator.
Unless the administrator determines otherwise, the restricted stock purchase agreement shall grant the Company a repurchase option exercisable upon the voluntary or involuntary termination of the purchaser’s employment with the Company for any reason (including death or disability). The purchase price for shares repurchased pursuant to the restricted stock purchase agreement shall be the original purchase price paid by the purchaser and may be paid by cancellation of any indebtedness of the purchaser to the Company. The repurchase option shall lapse at such rate as the administrator may determine.
Taxes. As a condition of the exercise of an option or stock purchase right granted under the Plan, the participant (or in the case of the participant’s death, the person exercising the option or stock purchase right) shall make such arrangements as the administrator may require for the satisfaction of any applicable federal, state, local or foreign withholding tax obligations that may arise in connection with the exercise of the option or stock purchase right and the issuance of shares. The Company shall not be required to issue any shares under the Plan until such obligations are satisfied. If permitted by the administrator, in its discretion, a participant may satisfy his or her tax withholding obligations upon exercise of an option or stock purchase right by surrendering to the Company shares that have a fair market value determined as of the applicable tax date equal to the amount required to be withheld.
Non-Transferability of Options and Stock Purchase Rights. Except as set forth in the Plan, options and stock purchase rights may not be sold, pledged, assigned, hypothecated, transferred or disposed of in any manner other than by will or by the laws of descent or distribution. Notwithstanding anything else, prior to the date, if any, on which the common stock becomes a listed security, the administrator may in its discretion grant nonstatutory stock options that may be transferred by instrument to an inter vivos or testamentary trust in which the options are to be passed to beneficiaries upon the death of the trustor (settlor) or by gift to “Immediate Family” (as defined below), on such terms and conditions as the administrator deems appropriate. Following the date, if any, on which the common stock becomes a listed security, the administrator may in its discretion grant transferable nonstatutory stock options pursuant to option agreements specifying the manner in which such nonstatutory stock options are transferable. “Immediate Family” means any child, stepchild, grandchild, parent, stepparent, grandparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, and shall include adoptive relationships.
Adjustments Upon Changes in Capitalization, Merger or Certain Other Transactions.
Change of Control. In the event of a Change of Control, (i) each outstanding option shall become immediately vested and exercisable, and (ii) any outstanding restricted stock shall become immediately vested and any repurchase option with respect to such restricted stock shall immediately lapse, in each case effective immediately prior to the Change of Control.”
Changes in Capitalization. Subject to any required action by the stockholders of the Company, the number of shares of common stock covered by each outstanding option or stock purchase right, the number of shares and the number of shares of common stock that have been authorized for issuance under the Plan but as to which no options or stock purchase rights have yet been granted or that have been returned to the Plan upon cancellation or expiration of an option or stock purchase right, as well as the price per share of common stock covered by each such outstanding option or stock purchase right, shall be proportionately adjusted for any increase or decrease in the number of issued shares of common stock resulting from a stock split, reverse stock split, stock dividend, combination, recapitalization or reclassification of the common stock, or any other increase or decrease in the number of issued shares of common stock effected without receipt of consideration by the Company; provided, however, that conversion of any convertible securities of the Company shall not be deemed to have been “effected without receipt of consideration.”
Dissolution or Liquidation. In the event of the dissolution or liquidation of our Company, each option and stock purchase right will terminate immediately prior to the consummation of such action, unless otherwise determined by the administrator.
| 35 |
Corporate Transaction. In the event of a Corporate Transaction, each outstanding option or stock purchase right shall be assumed or an equivalent option or right shall be substituted by such successor corporation or a parent or subsidiary of such successor corporation (the “Successor Corporation”), unless the Successor Corporation does not agree to assume the award or to substitute an equivalent option or right, in which case such option or stock purchase right shall terminate upon the consummation of the transaction in consideration for a cash payment to the participant (on the date of the Corporate Transaction), with respect to each such option, equal to the excess, if any, of the fair market value of the Common Stock subject to such option over the exercise price of such option.
Amendment and Termination of the Plan. The board may at any time amend, alter, suspend or discontinue the Plan, but no amendment, alteration, suspension or discontinuation shall be made that would materially and adversely affect the rights of any optionee or holder of stock purchase rights under any outstanding grant, without his or her consent. No amendment or termination of the Plan shall materially and adversely affect options or stock purchase rights already granted, unless mutually agreed otherwise between the optionee or holder of the stock purchase rights and the administrator, which agreement must be in writing and signed by the optionee or holder and the Company.
Securities Authorized for Issuance Under Equity Compensation Plans As of September 30, 2018
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining
available for future issuance | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 7,667,271 | $ | 1.70 | 9,481,065 | ||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| Total | 7,667,271 | $ | 1.70 | 9,481,065 | ||||||||
Recommendation of the Board of Directors
THE BOARD RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR” TO APPROVE AN AMENDMENT TO THE COMPANY’S 2013 AMENDED AND RESTATED STOCK PLAN, AS AMENDED, TO INCREASE THE NUMBER OF SHARES OF COMMON STOCK AUTHORIZED FOR ISSUANCE UNDER THE PLAN FROM 17,750,000 TO 22,750,000.
PROPOSAL 4
TO APPROVE AN AMENDMENT TO OUR CERTIFICATE OF INCORPORATION TO INCREASE THE NUMBER OF SHARES THE CORPORATION IS AUTHORIZED TO ISSUE TO 600,000,000 SHARES OF COMMON STOCK, PAR VALUE $0.001 PER SHARE
Shareholders are being asked to approve an amendment to the Company’s Certificate of Incorporation (the “Certificate”) to increase the number of authorized shares of Company common stock from four hundred million shares (400,000,000) to six hundred million shares (600,000,000). At its meeting held on September 28, 2018, the Board of Directors approved this amendment, subject to shareholder approval, and directed that this amendment be submitted to a vote of the Company’s shareholders at this Annual Meeting of Shareholders. The Board has determined that this amendment is in the best interests of the Company and its shareholders and recommends approval by the shareholders.
The Certificate currently authorizes the issuance of (i) up to 400,000,000 shares of Company common stock, each with a par value of $0.001 per share, and (ii) up to 50,000,000 shares of Company preferred stock, each with a par value of $0.001 per share. As of the close of business on November 5, 2018, 114,698,044 shares of common stock were outstanding and no shares of preferred stock were outstanding. In addition, as of the close of business on November 5, 2018, the Company had 7,667,271 shares of common stock subject to outstanding stock options and 9,481,065 shares reserved for issuance pursuant to future grants under the Company’s current stock incentive plans. The Company also had 43,534 shares reserved subject to the vesting of restricted stock. The Company also had 55,822,622 shares reserved subject to the exercise of outstanding warrants. This means that as of November 5, 2018, the Company had just 212,287,464 shares of common stock available for corporate purposes, including, among other things, the issuance of shares for potential financings.
| 36 |
Purpose of Amendment
The Board believes it is in the best interest of our Company to increase the number of authorized shares of common stock in order to give us greater flexibility in considering and planning for future potential business needs.
We have no current plan, commitment, arrangement, understanding or agreement regarding the issuance of the additional shares of common stock resulting from the proposed increase in authorized shares. The additional shares of common stock will be available for issuance by our Board for various corporate purposes, including but not limited to, stock splits, stock dividends, grants under employee stock plans, financings, potential strategic transactions, including mergers, acquisitions, strategic partnerships, joint ventures, divestitures, and business combinations, as well as other general corporate transactions.
Having this additional authorized common stock available for future use will allow us to issue additional shares of common stock without the expense and delay of arranging a special meeting of shareholders.
Possible Effects of the Amendment and Additional Anti-takeover Consideration
If the amendment to the Certificate is approved, the additional authorized shares would be available for issuance at the discretion of our Board and without further shareholder approval, except as may be required by law. The additional shares of authorized common stock would have the same rights and privileges as the shares of common stock currently issued and outstanding. There are currently no shares of preferred stock outstanding. The adoption of the amendment would not have any immediate dilutive effect on the proportionate voting power or other rights of existing shareholders. Shares of common stock issued otherwise than for a stock split may decrease existing shareholders’ percentage equity ownership and, depending on the price at which they are issued, could be dilutive to the voting rights of existing shareholders and have a negative effect on the market price of the common stock. Current shareholders have no preemptive or similar rights.
We cannot provide assurances that any such transactions will be consummated on favorable terms or at all, that they will enhance shareholder value or that they will not adversely affect our business or the trading price of our stock.
We have not proposed the increase in the number of authorized shares of common stock with the intention of using the additional authorized shares for anti-takeover purposes, but we would be able to use the additional shares to oppose a hostile takeover attempt or delay or prevent changes in control or management. For example, without further shareholder approval, our Board could sell shares of common stock in a private transaction to purchasers who would oppose a takeover or favor our current Board. Although this proposal to increase the authorized number of shares of common stock has been prompted by business and financial considerations and not by the threat of any known or threatened hostile takeover attempt, shareholders should be aware that approval of this proposal could facilitate future efforts by us to oppose changes in control of our Company and perpetuate our management, including transactions in which the shareholders might otherwise receive a premium for their shares over then current market prices.
If our shareholders approve the increase in the number of authorized shares of common stock to 400,000,000, the Board will have authority to file with the Secretary of State of Delaware an amendment to our Certificate, effectively increasing our authorized shares by an additional 200,000,000 shares of common stock. Upon approval and following such filing with the Secretary of State of the State of Delaware, the amendment will become effective on the date it is filed. The amendment proposed by us to the Article FOURTH of our Certificate is attached to this proxy statement as Appendix B.
Neither Delaware law, our Certificate, nor our by-laws provides for appraisal or other similar rights for dissenting shareholders in connection with this proposal. Accordingly, our shareholders will have no right to dissent and obtain payment for their shares.
| 37 |
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS A VOTE FOR THE AMENDMENT OF THE COMPANY’S CERTIFICATE OF INCORPORATION TO INCREASE THE NUMBER OF AUTHORIZED SHARES.
OTHER MATTERS
As of the date hereof, there are no other matters that we intend to present, or have reason to believe others will present, at the annual meeting. If, however, other matters properly come before the annual meeting, the accompanying proxy authorizes the person named as proxy or his substitute to vote on such matters as he determines appropriate.
ANNUAL REPORT ON FORM 10-K
As required, we filed our 2017 Form 10-K, as amended, with the SEC. Stockholders may obtain, free of charge, a copy of the 2017 Form 10-K by writing to us at Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York NY 10016, Attention: Corporate Secretary, or from our website, www.actiniumpharma.com under the heading “Investor Relations” and the subheading “Company Financial Reports.”
HOUSEHOLDING OF PROXY MATERIALS
SEC rules concerning the delivery of annual disclosure documents allow us or your broker to send a single Notice or, if applicable, a single set of our proxy materials to any household at which two or more of our stockholders reside, if we or your broker believe that the stockholders are members of the same family. This practice, referred to as “householding,” benefits both you and us. It reduces the volume of duplicate information received at your household and helps us to reduce our expenses. The rule applies to our Notices, annual reports, proxy statements and information statements. Once you receive notice from your broker or from us that communications to your address will be “householded,” the practice will continue until you are otherwise notified or until you revoke your consent to the practice. Stockholders who participate in householding will continue to have access to and utilize separate proxy voting instructions.
If your household received a single Notice or, if applicable, a single set of proxy materials this year, but you would prefer to receive your own copy, please contact Alliance Advisors, by calling their toll free number, 1-877-777-2857.
If you do not wish to participate in “householding” and would like to receive your own Notice or, if applicable, set of our annual disclosure documents in future years, follow the instructions described below. Conversely, if you share an address with another holder of our Common Stock or Preferred Stock and together both of you would like to receive only a single Notice or, if applicable, set of our annual disclosure documents, follow these instructions:
If your shares are registered in your own name, please contact Alliance Advisors, and inform them of your request by calling them at 1-877-777-2857 or writing them at 200 Broadacres Drive, 3rd Fl., Bloomfield, NJ 07003
If a broker, bank or other nominee holds your shares, please contact the broker, bank or other nominee directly and inform them of your request. Be sure to include your name, the name of your brokerage firm and your account number.
Electronic Delivery of Company Stockholder Communications
Most stockholders can elect to view future proxy statements and annual reports over the Internet instead of receiving paper copies in the mail.
You can choose this option and save the cost of producing and mailing these documents by:
following the instructions provided on your Notice or proxy card;
following the instructions provided when you submit a proxy to vote over the Internet; or
going to www.AALvote.com/atnm.com and following the instructions provided.
| 38 |
IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE STOCKHOLDER MEETING TO BE HELD ON DECEMBER 20, 2018
This proxy statement and our 2017 Form 10-K to stockholders are available for viewing, printing and downloading at http://www.viewproxy.com/actinium pharma/2018 or by email at: requests@viewproxy.com. To view these materials, please have your control number available that appears on your Notice or proxy card. On this website, you can also elect to receive future distributions of our proxy statements and annual reports to stockholders by electronic delivery.
Additionally, you can find a copy of our Annual Report on Form 10-K, which includes our financial statements, for the fiscal year ended December 31, 2017 on the website of the Securities and Exchange Commission, or the SEC, at www.sec.gov, or in the “SEC Filings” section of the “Investors & Media” section of our website at www.actiniumpharma.com. You may also obtain a printed copy of our Annual Report on Form 10-K, including our financial statements, free of charge, from us by sending a written request to: Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York, NY 10016, attention: Principal Financial Officer. Exhibits will be provided upon written request and payment of an appropriate processing fee.
PROPOSALS OF STOCKHOLDERS
Stockholders may present proposals intended for inclusion in our proxy statement for our 2019 Annual Meeting of Stockholders provided that such proposals are received by the Secretary of the Company in accordance with the time schedules set forth in, and otherwise in compliance with, applicable SEC regulations, and the Company’s Amended and Restated Bylaws, as applicable. Proposals submitted not in accordance with such regulations will be deemed untimely or otherwise deficient; however, the Company will have discretionary authority to include such proposals in the 2018 Proxy Statement.
WHERE YOU CAN FIND MORE INFORMATION
This proxy statement refers to certain documents that are not presented herein or delivered herewith. Such documents are available to any person, including any beneficial owner of our shares, to whom this proxy statement is delivered upon oral or written request, without charge. Requests for such documents should be directed to Principal Financial Officer, Actinium Pharmaceuticals, Inc., 275 Madison Avenue, 7th Floor, New York, NY 10016, (646) 677-3875. Please note that additional information can be obtained from our website at www.actiniumpharma.com.
We file annual and special reports and other information with the SEC. Certain of our SEC filings are available over the Internet at the SEC’s web site at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities:
Public Reference Room Office 100 F Street,
N.E.
Room 1580
Washington, D.C. 20549
You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Callers in the United States can also call 1-202-551-8090 for further information on the operations of the public reference facilities.
| 39 |
Annex A
AMENDMENT NO. 6
TO
ACTINIUM PHARMACEUTICALS, INC. 2013 AMENDED AND RESTATED STOCK PLAN
Pursuant to Section 14 of the 2013 Amended and Restated Stock Plan, as amended (the “Plan”) of Actinium Pharmaceuticals, Inc. (the “Company”), the Board of Directors of the Company has duly adopted a resolution, conditioned upon approval by the stockholders of the Company, approving this Amendment No. 6 to the Plan to increase the total number of shares of common stock, par value $.001 per share, of the Company (the “Common Stock”) reserved and available for issuance under the Plan as follows:
| 1. | Section 3 of the Plan is hereby amended to read in its entirety as follows: |
“Subject to the provisions of Section 14 of the Plan, the maximum aggregate number of Shares reserved for issuance to Participants under the Plan is 22,750,000, and the maximum aggregate number of Shares that may be granted in the form of Incentive Stock Options is 22,750,000. The Shares may be authorized, but unissued, or reacquired Common Stock. If an award should expire or become unexercisable for any reason without having been exercised in full, or is surrendered pursuant to an Option Exchange Program, the unpurchased Shares that were subject thereto shall, unless the Plan shall have been terminated, become available for future grant under the Plan. In addition, any Shares of Common Stock which are retained by the Company upon exercise of an award in order to satisfy the exercise or purchase price for such award or any withholding taxes due with respect to such exercise or purchase shall be treated as not issued and shall continue to be available under the Plan. Shares issued under the Plan and later repurchased by the Company pursuant to any repurchase right which the Company may have shall not be available for future grant under the Plan.”
| 2. | All other terms and provisions of the Plan shall remain unchanged and in full force and effect as written. |
| 3. | A majority in voting interest of the stockholders present in person or by proxy and entitled to vote at the meeting of stockholders at which this Amendment No. 6 was considered, has duly approved this Amendment No. 6 to the Plan. |
IN WITNESS WHEREOF, this Amendment No. 5 to the Plan is made effective this day of , 2018.
| ACTINIUM PHARMACEUTICALS, INC. | ||
| By: | ||
| A-1 |
Appendix B
CERTIFICATE OF AMENDMENT TO
CERTIFICATE OF INCORPORATION
OF ACTINIUM PHARMACEUTICALS, INC.
Actinium Pharmaceuticals, Inc., a Delaware corporation, hereby certifies that:
| 1. | The Certificate of Incorporation is amended (a) by replacing Article FOURTH thereof to read in its entirety as follows: |
Article FOURTH
“FOURTH: The amount of the total stock this Corporation is authorized to issue is 650,000,000 shares with a par value of $0.001 per share.
(a) Common Stock. The aggregate number of shares of Common Stock which the Corporation shall have authority to issue is 600,000,000 shares at a par value of $0.001 per share.
(b) Preferred Stock. The aggregate number of shares of Preferred Stock which the corporation shall have authority to issue is 50,000,000 shares, par value $0.001, which may be issued in series, with such designations, preferences, stated values, rights, qualifications or limitations as determined solely by the Board of Directors of the Corporation.
(c) Preemptive rights. No stockholder of the Corporation shall have any preemptive right to subscribe to an additional issue of stock or to any security convertible into such stock of the Corporation.”
| 2. | This Certificate of Amendment has been duly adopted in accordance with Section 242 of the General Corporation Law of the State of Delaware (the “DGCL”), including approval by the stockholders of the corporation upon notice in accordance with Section 222 of the DGCL, at the annual meeting of the stockholders on the Corporation held on December 18, 2018. |
IN WITNESS WHEREOF, the corporation has caused this certificate to be signed by its duly authorized officer this __ day of December, 2018.
Sandesh Seth, Chairman and Chief Executive Officer
| B-1 |

