
Exhibit 99.1

Letter to Investors
Company Update and Outlook for 2018
On behalf of the team at Actinium, I would like to wish you all an awesome 2018 and also thank our many shareholders for their unstinting support of the Company’s proposals for the Annual Shareholder Meeting in December, all of which passed with resounding majorities.
After bearing witness to the Company’s performance over the past several years as a board member and Executive Chairman, I have confidence stating that today, Actinium, from an operations perspective, has broken from its past. The several news releases in December provide a visible signal of the capabilities of our new team who have set the foundation for an event driven 2018 that can result in significant value creation. I paraphrase below italicized language from the attached fact sheet which sums up our transformation from dysfunction to function:
| - | Clinical operations are now fully functional with over fifty patients enrolled across all 3 clinical trials as of early December with the overwhelming majority enrolled after June 2017. |
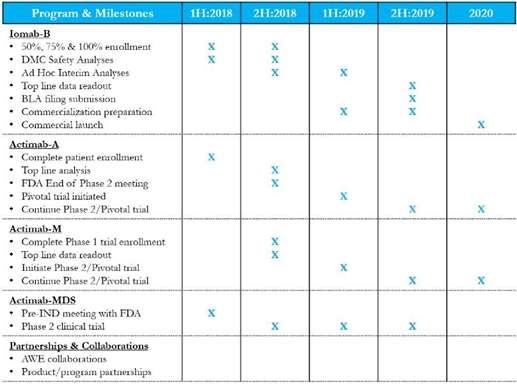
| ● | Enrollment of Iomab-B in our pivotal SIERRA trial is proceeding well and is on track for the significant clinical milestones expected in 2018. |
| ● | Enrollment of Actimab-A which was lackluster compared to industry standards in years prior is now robust despite 4 recent product approvals in AML and this trial is on track for topline results in 2018 as per prior guidance. |
| - | The Company has effectively set up the exoskeleton of a commercial infrastructure at this time in terms of supply chain, across all 3 clinical programs. | |
| - | Importantly, our team has leveraged the myelosuppressive capability of the CD33 targeting construct into a potentially transformative clinical initiative in MDS or Myelodysplastic Syndromes via Actimab-MDS. | |
| - | Actimab-MDS provides the Company another product candidate in the bone marrow transplant market. | |
| - | Actimab-M provides another shot on goal for Actinium’s CD33 Program and bolsters our claim that this program has best-in-class potential. | |
| - | The launch of the AWE Program is designed to build on these results for internal purposes and collaborations. Our new Chief Scientific Officer, Dr. Dale Ludwig is expected to add material value to these efforts going forward. |
I would also like to provide some perspective on our programs and the progress we have made last year, most of it under the direction of our new management team.
| 1 |
Building an Industry Leading CD33 Program with Multiple Shots on Goal
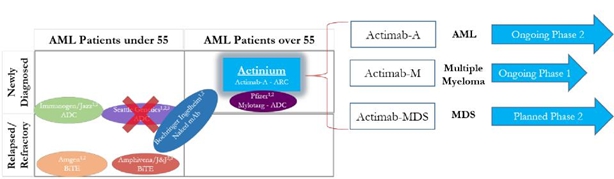
The Company is developing, potentially, the leading CD33 targeting program in the industry with clinical programs in three indications:
| - | Actimab-A for unfit elderly AML patients in Phase 2 | |
| - | Actimab-M for refractory multiple myeloma in a proof of concept Phase 1 trial | |
| - | Actimab-MDS for BMT conditioning in high-risk MDS patients with a planned Phase 2 |
As illustrated in the diagram below, there are several large biotech/pharma companies developing drug candidates targeting CD33 but only in AML as they may be limited by their technological approach. Actinium is able to pursue multiple indications because our ARC or Antibody Radiation Conjugate approach has several advantages:
| - | The radioisotope can destroy cellular DNA from the cell surface due to linear energy transfer, so an ARC does not need to internalize. This capability is important as it translates to lower drug concentrations required for potency and also lower target expression. | |
| - | ARCs
can also destroy cells by crossfire which further increases their potency. This ability provides a viable approach in diseases outside of AML like multiple myeloma and MDS. ADCs, bi-specifics and naked antibodies are unlikely to have the same potential. | |
| - | Potency
of the ARC and low amounts of antibody required for effective targeting translate to relative ease of administration, a safety benefit, monotherapy potential and treatment on an outpatient basis. |
Actinium’s CD33 program should yield multiple clinical milestones in 2018 that can solidify its leadership potential.
Actinium’s CD33 program is the only unpartnered program developed by a smaller company.
I am excited about the progress we have made under this program. Our Chief Medical Officer, Dr. Mark Berger, is something of a CD33 expert who was responsible for the first approval of Mylotarg. Under his stewardship, enrollment in our lead trial for Actimab-A is proceeding well and topline results are expected by mid-year. Actimab-M and Actimab-MDS are also being well executed and I believe that in 2018 we will progress the CD33 program to a point where its superiority in the class is well established.
| 2 |
Unlocking Significant Value in Myeloablation for Bone Marrow Transplant
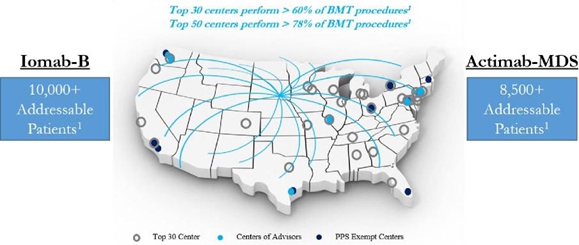
Bone Marrow Transplant or BMT is an accepted way of treating cancer patients that can result in cures. This area of medicine while well-established is not well-serviced by biopharma companies and consequently not well understood by investors as there are very few companies that focus on improving BMT. Lately the area has been drawing some attention by “smart money” venture investors and the investment banks that service them, as companies are beginning to focus on improving various aspects of BMT. Also, certain analysts and investors are starting to realize that BMT offers a successful and well-established pathway for curing cancer and are making insightful comparisons with the currently more limited CAR-T therapy which should help bring more attention to this area of medicine.
Iomab-B, our CD45 targeting ARC and Actimab-MDS are being developed to provide enhanced access to bone marrow transplant, with improved transplant outcomes via improved myeloablation or conditioning of the bone marrow prior to a transplant. In plain English, these drug candidates wipe out the diseased bone marrow effectively but in a much safer way than the highly toxic chemotherapy regimens that are current standard of care. As a result of this effect, patients that cannot withstand chemotherapy can go to transplant with our drug candidates and have better results from the procedure including cures. Actimab-M also has that potential in transplant.
The concentrated nature of centers providing bone marrows transplant positions Actinium uniquely to serve this major unmet medical need with the progress made in 2H:2017. Our hard-won relationships with key transplant centers, understanding of internal protocols governing use of ARCs and transplant, a well-functioning supply chain under the leadership of Dr. Nitya Ray, and the simple fact that the prior data in hundreds of patients treated with our drug candidates attracts investigators to participate in our programs, provides the foundation for realizing this vision and making material strides towards that goal in 2018. Important to note that we are the only company with programs that are as advanced, and we intend to extend our leadership position this year.
Actinium
is unique in that there is no other company with a multi-disease, multi-product
pipeline that targets improved transplant access and outcomes via improved myeloablation.
| 3 |
Company Outlook
In a nutshell, recent events send a clear signal that the Company is now fully functional, able to operate efficiently on all fronts, and recruit high quality talent. Indeed, we are now capable of driving toward the milestones of 2018 that have the potential for significant value accretion from our 4 clinical programs and the AWE Program. Assuming that the early signs bear themselves out and yield positive results, significant and transformative value creation is possible.
Our executive team is committed to generating value by achieving the milestones above. Our entire company is motivated and energized to materialize our shared vision for Actinium. Our intent over the next three to five years is to build upon the promise of both the CD45 and CD33 programs by gaining approval and commercializing multiple product candidates based on these programs to enhance access to bone marrow transplant with improved outcomes via our improved myeloablation approach and to partner strategically to enhance the value of our programs.
We look forward to providing both company and program updates throughout 2018 as we work both smartly and tirelessly to succeed in this endeavor.
| Respectfully, | |
| /s/ Sandesh Seth | |
| Sandesh Seth | |
| Chairman and CEO |
4