Exhibit 10.17
CLINICAL TRIAL AGREEMENT
THIS AGREEMENT is made and entered into by and between SLOAN-KETTERING INSTITUTE FOR CANCER RESEARCH and its affiliate MEMORIAL HOSPITAL FOR CANCER AND ALLIED DISEASES both having a principal place of business at 1275 York Avenue, New York, New York 10065, membership corporations of the State of New York (hereinafter " SKI/MEMORIAL"), and ACTINIUM PHARMACEUTICAL INC., a corporation having its principal place of business at 391 Lafayette Street, Newark, NJ 07105 (hereinafter "COMPANY"). This Agreement is effective as of the date of the last party to subscribe below (hereinafter "Effective Date").
WITNESSETH
WHEREAS, SKI/MEMORIAL will conduct a clinical trial entitled "A Phase I/II Study of Low Dose Cytarabine and Lintuzumab-Ac225 in Older Patients with Untreated Acute Myeloid Leukemia " (IND # 10807), hereinafter "Study"), which is a clinical investigation using a proprietary drug which is not, at this time, cleared for human use by the U.S. Food and Drug Administration ("FDA"). Any use of this drug must be pursuant to an Investigational New Drug Exemption issued to Company by the FDA, and
WHEREAS, Aptiv Solutions, Inc., a Delaware corporation having a principal place of business at 1925 Isaac Newton Square, Suite 100, Reston, VA 20190 ("Aptiv") has been engaged by Company to oversee and manage the Study and
WHEREAS, COMPANY conducts business in the development, manufacture and sale of therapeutic products, and is interested in sponsoring the Study in exchange for access to the data resulting from the Study.
NOW, THEREFORE intending to be legally bound and upon the terms, conditions and covenants hereinafter set forth, SKI/MEMORIAL and COMPANY agree as follows:
ARTICLE 1-THE STUDY
1.1 SKI/MEMORIAL has established and maintains a Leukemia Service, a Division of Hematologic Oncology, in the Department of Medicine and has acquired expertise in conducting research investigations, clinical trials and laboratory test evaluations.
1.2 The Study under this Agreement will be conducted under a protocol approved by SKI/MEMORIAL'S Human Subject Institutional Review Board (hereinafter "IRB"), based on the draft protocol annexed hereto as Exhibit A (hereinafter "Protocol"). SKI/MEMORIAL shall submit the Protocol for approval to the IRB and Company shall submit Protocol to the FDA. COMPANY shall supply Lintuzumab-Actinium-225 after COMPANY has:
a. received a mutually executed copy of this Agreement;
b. received documentation from SKI/MEMORIAL that SKI/MEMORIAL'S IRB has approved the Protocol.
Promptly after SKI/MEMORIAL'S IRB has approved the Protocol, SKI/MEMORIAL shall forward a copy as approved to COMPANY. SKI/MEMORIAL shall also forward any subsequent change to the Protocol to COMPANY. Except for Protocol changes required by the FDA, all Protocol changes must be approved by COMPANY.
1.3 As part of this Agreement, SKI/MEMORIAL shall appoint Joseph G. Jurcic, M.D. and/or such other physicians as it may deem appropriate as investigators (hereinafter "Investigators") to oversee the Study. If Dr. Jurcic should become unable to complete the Study, SKI/MEMORIAL shall consult with COMPANY regarding the appointment of a new principal investigator.
1.4 The Investigators on behalf of SKI/MEMORIAL shall prepare and maintain records and case histories with all pertinent data documented as required by the Protocol on case report forms supplied by COMPANY. The parties shall hold all patient data confidential, and information provided to COMPANY shall not disclose patient health information, except to the extent that the patient consent form permits. COMPANY may disclose reports and other information to Aptiv and to an independent data management company, provided Aptly and the management company is bound to hold such information in confidence.
1.5 The Investigators shall also promptly notify COMPANY, Aptiv and the IRB of any adverse reaction in the course of the Study of which they become aware, but in no event shall such notice be later than twenty-four (24) hours after each occurrence of an adverse, serious or unexpected event, or any deviation in the Protocol permitted by 21 CFR 312.60(a)(2). The Investigator shall complete all reports when and in the manner required by 21 CFR 312.62 and 312.64. The Investigator shall make all other reports as required by 2.1 CFR 312.62 and 312.64
1.6 SKI/MEMORIAL and COMPANY agree that in the performance and documentation of the Study they shall adhere to this Agreement, the Protocol and all applicable government laws, rules, regulations and guidelines, including but without limitation the Health Insurance Portability and Accountability Act of 1996 ("HIPAA") and its regulations and official guidance promulgated thereunder, and those of the FDA, including among others the Generic Drug Enforcement Act of 1992 (21 USC §§ 305,306). Certifications and other documents required by these statutes and regulations, such as those relating to financial conflicts of interest and debarment from performing clinical trials, shall be provided as necessary.
1.7 COMPANY shall provide SKI/MEMORIAL with any investigational protocols, preclinical or background information which are germane to the Study.
1.8 Upon SKI/MEMORIAL'S request, COMPANY shall provide, without cost to SKI/MEMORIAL, sufficient amounts of Lintuzumab-Actinium-225 to conduct the Study.
1.9 SKI/MEMORIAL shall permit COMPANY and Aptiv to monitor the progress of the Study through site visits and review of Study reports and related documentation. The parties agree that COMPANY may engage other third parties of COMPANY'S choosing to conduct the Study monitoring.
1.10 SKI/MEMORIAL shall provide the physician, laboratory, statistical and clinical support staff levels of effort required to complete the Study
ARTICLE II — REPORTS
2.1 SKUMEMORIAL shall keep COMPANY and Aptiv advised of the status of the Study via periodic reports. The frequency of reports shall be mutually agreed to by both parties. At any time all case reopt forms and other Study data shall be submitted to Aptiv within ten (10) days of written request. There shall also be a final report of the Study presented to COMPANY and Aptiv within sixty (60) days of the Study completion.
2.2 All reports submitted to COMPANY or Aptiv shall become the property of COMPANY and may be used by COMPANY for its internal uses and for use in communications with the FDA and other regulatory authorities, filing of patent applications by the Company, and otherwise as required by law. If COMPANY desires to release the reports or any contents in the reports to the public domain by any means or methods such as press releases, publications, meeting presentations, COMPANY must first obtain written consent from SKI/MEMORIAL, which consent will not be unreasonably withheld or delayed. Notwithstanding the above, COMPANY shall not release any reports or any contents that could jeopardize publication under Article III.
ARTICLE III — PUBLICATION
Notwithstanding anything contained herein to the contrary including without limitation Article IV, but subject to the provisions of this Article III, SKI/MEMORIAL may freely publish the results of its investigative findings hereunder. The authorship and contents (including scientific conclusions and professional judgments) of any paper submitted shall be determined by SKI/MEMORIAL. SKI/MEMORIAL shall provide COMPANY with a copy of the papers prepared for publication prior to their submission to a scientific journal or presentation at scientific meetings. COMPANY shall have thirty (30) days to review the papers. COMPANY shall not make any editorial changes in the papers, but may delete any of its Confidential Information (as defined in Article V) contained therein. COMPANY personnel shall be acknowledged with customary scientific practice. The parties recognize that because this is a multicenter Study, there is a need for a coordinated approach to any publication or public disclosure of the data or results of this Study. To that end, there will be no publication or public disclosure of such data or results by SKI/MEMORIAL or Investigator until a multi-center publication is submitted for publication or presentation by Actinium, or its designee. However, if no multi-site publication is submitted by Actinium or its designee within twelve (12) months of the completion of the Study from all sites, SKI/MEMORIAL and the Investigator shall be free to publish for non-commercial purposes the Study results in accordance with this Article III.
ARTICLE IV - CONFIDENTIAL INFORMATION
4.1 In order to effectively complete the Study, it may be necessary or desirable for the parties to disclose proprietary, trade secret and/or information relating to patients (hereinafter "Confidential Information") to one another.
4.1.1 All medical records (or other patient infoiation) not transcribed into the case report forms are Confidential Information of SKI/MEMORIAL, and do not need to be marked "Confidential". There shall be no time limit on the parties' obligation to maintain the confidentiality of patient identifiable health information, including information whose identifiers may be ascertained by the exercise of reasonable effort through investigation. Patient identifiable health information shall be protected in compliance with all applicable regulations, rules and statutes. COMPANY agrees to refrain from publishing or disclosing any part of such confidential medical records or from using it except as necessary to discuss and analyze the results of the Study, to ensure research integrity, to communicate with the FDA and other regulatory authorities, and otherwise as required by law or specifically permitted by authorizations or consents signed by Study subjects, or waivers of authorization granted by an IRB overseeing the Study ("Permitted Activities"). COMPANY also agrees to restrict the use and disclosure of any individually identifiable health information gained through the Permitted Activities to its workforce, contractors, subcontractors, Study collaborators and agents who must have access to that information in order directly to support or facilitate the Permitted Activities, and to use the necessary means to bind those parties to these restrictions and requirements, as though these restrictions and requirements applied to these entities directly.
4.1.2 Any other Confidential Information shall be marked as "Confidential" or, if provided to the other party orally. shall be reduced to writing marked as "Confidential" and sent to the other party within ten (10) days of the oral disclosure, except that this requirement shall not apply to patient information, which is always Confidential Information. Each party agrees that such other Confidential Information of the other party disclosed to it or to its employees or an independent data management company shall for three (3) years after disclosure:
|
a)
|
be used only in connection with the legitimate purposes of this Agreement;
|
|
b)
|
be disclosed only to those who have a need to know it; and
|
|
c)
|
be safeguarded with the same care normally afforded confidential information in the possession, custody or control of the party holding the Confidential Information.
|
The foregoing shall not apply when, after and to the extent the Confidential Infatuation Disclosed:
|
i.
|
can be demonstrated to have been in the public domain prior to the date of the disclosure; or
|
|
ii.
|
enters the public domain through no fault of the receiving party; or
|
|
iii.
|
was already known to the receiving party at the time of disclosure as evidenced by written records in the possession of the receiving party prior to such time; or
|
|
iv.
|
is subsequently received by the receiving party in good faith from a third party without breaching any confidential obligation between the third party and the disclosing party; or
|
|
v.
|
was independently developed, as established by tangible evidence, by the receiving party without reference to information or material provided by the disclosing party; or
|
|
vi.
|
is required to be disclosed for minimal compliance with court orders, statutes or regulations or SKI/MEMORIAL audits for compliance with such regulatory requirements, provided that prior to any such disclosure to the extent reasonably practicable, the party from whom disclosure is sought shall promptly notify the other party and shall afford such other party the opportunity to challenge or otherwise lawfully seek limits upon such disclosure of Confidential Information.
|
ARTICLE V — COMPENSATION
5.1 Definition:
For purposes of this Agreement, the following definitions apply:
| |
i.
|
Screening is the process of identifying potential subjects according to the entrance criteria outlined in the Protocol and of conducting the examinations and test specified in the Protocol necessary to select qualified subjects for the Study. For any given subject, the screen phase ends and the treatment phase begins when the subject has been enrolled into a treatment group under the Protocol and has been dispensed any Study Drug or placebo by SKI/MEMORIAL according to the study design and regimen described in the Protocol.
|
| |
ii.
|
A qualified subject is one who, upon entrance into the treatment phase of the Study, met all of the entrance criteria and none of the exclusion criteria in the Protocol and for whom knowing, written informed consent to participate was obtained in accordance with sub-Article 5.1.i herein.
|
| |
iii.
|
A completed subject is a qualified subject who completed the full term of the Study and met the minimum attendance and compliance standards in the Protocol so that the Study Drug can be evaluated for safety or effectiveness. A completed case report form is a set of Clinical Research Database (CRDB) generated reports submitted to the COMPANY for a completed subject which meets the requirements set forth in the Protocol.
|
| |
iv.
|
An incomplete subject is a qualified subject who started the Study but failed to complete the Protocol satisfactorily because of insufficient clinic attendance, poor compliance, voluntary withdrawal, or other violations of the Protocol.
|
5.2 Amounts of Payment
The compensation to SKI/MEMORIAL for the Study shall be calculated as follows:
| |
i.
|
For each completed case report form (set OI CRDB generated reports) on a completed subject, COMPANY will pay thirty one thousand one hundred eighty five U.S. Dollars ($31,185).
|
| |
ii.
|
For each case report (CRDB generated report) on an incomplete subject, the COMPANY will pay on a pro-rated basis taking into account that the initialization of the treatment maybe more expensive.
|
| |
iii.
|
SKI/MEMORIAL will receive no compensation for the examinations and tests conducted in non-qualified subjects under the screening or treatment phases of the Protocol.
|
| |
iv.
|
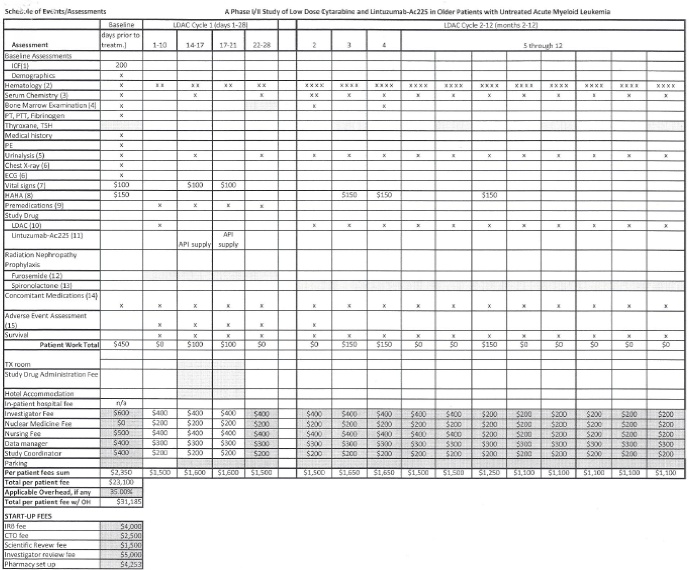
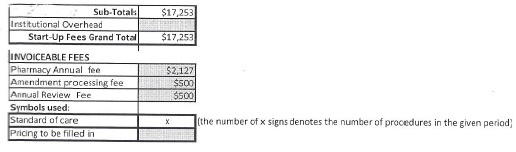
A start-up fee of seventy-nine thousand six hundred and twenty three dollars ($79,623) that includes $17,253 to cover costs required to initiate the study and the remainder to cover study costs through the submission of the first two case reports.
|
5.3 Other Conditions of Payment
| |
i.
|
SKI/MEMORIAL agrees to use reasonable efforts in accordance with industry custom and practice to follow the Protocol, recruit, screen and enroll qualified subjects, prepare case report forms and any reports required in the Protocol.
|
| |
ii.
|
The parties agree that the screening phase will begin as soon as practicable after the signing of this Agreement. Each subject will be considered to be a completed, or incomplete patient, as appropriate, only after SKI/MEMORIAL has produced all completed case report forms (set of CRDB generated reports) and COMPANY has accepted the completed case report forms (set of CRDB generated reports).
|
| |
iii.
|
In the event the Study is terminated prior to the anticipated Study completion date as described in Article VII herein, COMPANY will pay actual expenses incurred by SKI/MEMORIAL for all completed and incomplete patients accrued to the date of termination, as detailed above. If the Study is terminated by COMPANY prior to the planned completion date for its convenience and without cause, COMPANY agrees to pay for those subjects active in the treatment phase at the termination date as if they had completed the Study.
|
| |
iv.
|
The dates and financial arrangements in this Agreement can be modified only by written amendment to this Agreement signed and executed by both parties.
|
5.4 Schedule of Payment
In consideration for SKI/MEMORIAL'S participation and to cover a portion of the costs associated with the Study, COMPANY shall pay SKI/MEMORIAL as follows:
|
a.
|
$79,623 within thirty (30) days after the execution of this Agreement; and
|
|
b.
|
the remainder, as outlined in Sections 5.2 and 5.3, upon COMPANY'S receipt of final case reports (set of CRDB generated reports) on each group of 3 of the patients enrolled into the Study in accordance with the Protocol. SKI/MEMORIAL anticipates enrolling a total of 15 patients.
|
The above payments shall constitute full and final compensation to SKI/MEMORIAL under this Agreement unless agreed otherwise in writing by both parties.
5.5 SKI/MEMORIAL shall discuss if COMPANY so requests, budgetary matters with COMPANY, but reserves the right to be the final control on budgetary categories and expenditures.
The checks shall be made payable to Sloan-Kettering Institute for Cancer Research (Sloan Kettering Institute Tax I.D. No. 13-1624182) and shall be forwarded to:
Memorial Sloan-Kettering Cancer Center.
P. 0. Box 29049
New York, New York 10087-9035
COMPANY should note on its check stub or in its transmittal letter that the payment relates to a Clinical Trial Agreement, SK2011-1346, under the direction of Dr. Jurcic.
ARTICLE VI -INDEPENDENT CONTRACTOR
Both parties shall, at all times during the performance of this Agreement, remain as independent contractors and the Agreement shall not make the parties partners, joint venturers, or agents of one another. No party to this Agreement shall have the power to bind or obligate the other party
ARTICLE VII - TERM AND TERMINATION
7.1 This Agreement shall commence on the Effective Date of this Agreement and shall continue until
completion as provided in the Protocol, which is estimated to occur twenty four (24) months from the Effective Date hereof.
7.2 This Agreement can be terminated by either SKI/MEMORIAL or COMPANY with or
without cause upon thirty (30) days prior written notice without penalty to either party. Notwithstanding any notice period, SKI/MEMORIAL may immediately cease provision of services pursuant to the Protocol if either the Principal Investigator or the IRB determines that immediate cessation is appropriate for patient safety.
7.3 In the event that this Agreement is terminated by COMPANY or for safety reasons prior to
completion of the Study, the amount due to SKI/MEMORIAL from COMPANY shall be $ 31,185.00 for each patient who was enrolled in the Study any time between the Effective Date and the date of termination of this Agreement. For purposes of this Agreement enrollment shall mean a patient that has signed the .IRBapproved patient Informed Consent Form for the Study and successfully passed any pretreatment screening that is required.
7.4 If COMPANY terminates the Agreement prior to completion of the Study, COMPANY shall, if permitted by law and requested by SKI/MEMORIAL, supply SKI/MEMORIAL, free of charge, with sufficient Study Material to allow SKI/MEMORIAL to complete the treatment of those patients participating in the Study on the date of SKI/MEMORIAL'S receipt of COMPANY'S telmination notice.
7.5 Sections 1.5, 7.4, 7.5, 11.1-11.5 and Articles IL III, IV, VII, IX, and X shall all survive the termination of this Agreement.
ARTICLE VIII - REPRESENTATIONS AND WARRANTIES
8.1 SKI/MEMORIAL represents and warrants to COMPANY that:
| |
i.
|
all aspects of SKI/MEMORIAL'S facilities which may be used in the performance of the Study have been fully validated and are in compliance with applicable federal, state and local governmental requirements; it will obtain IRB review and approval of informed consent documentation, Study Protocol, and other relevant documentation prior to initiation of the Study;
|
| |
ii.
|
it will adhere to all aspects of the Protocol, including but not limited to patient enrollment criteria:
|
| |
iii.
|
SKI/MEMORIAL will receive no compensation for the examinations and tests conducted in non-qualified subjects under the screening or treatment phases of the Protocol.
|
| |
iv.
|
it will maintain proper control and inventory over the Actinium-225 and Study Drug. 7
|
| |
vi.
|
Investigators are employees of SKI/MEMORIAL, and are sufficiently qualified by training and experience to conduct the Study and have never been involved in any investigation or research at SKIIMEMORIAL which was terminated by the FDA, National Institutes of Health (NIB) or any sponsor for non-compliance.
|
8.2 COMPANY represents and warrants to SKI/MEMORIAL that it is authorized to enter into this Agreement, to provide Lintuzumab — Actinium 225 under this Agreement and that its execution, delivery and performance of this Agreement will not conflict with or constitute a default under any other agreement to which it is a party or by which its assets are bound.
8.3 COMPANY represents and warrants that it will not obtain any portion of the Lintuzumab from Protein Design Labs, Inc. (hereinafter "PDL ") or its successors in interest.
8.4 COMPANY and SKI/MEMORIAL represent and warrant to each other that:
(i) Neither they nor their employees, agents and subcontractors who provide services in connection with this Agreement have been excluded from participation in, or otherwise sanctioned by Medicare, Medicaid or any other federal, state or local health care program, and will promptly notify the other party if it or any such entity becomes so excluded or sanctioned during the term of this Agreement.
(ii) They have not been found by the FDA or any other state or federal government agency or enforcement body to have violated any relevant federal, state or local laws, rules or regulations relating to clinical investigations. If it is so found during the term of this Agreement, whether in connection with the Study, or in connection with any other clinical investigations or studies, the party so informed will notify the other party immediately.
ARTICLE IX - OWNERSHIP RIGHTS
9.1 Preservation of Data. Notwithstanding anything else in this Agreement to the contrary, unless specifically instructed otherwise in writing by COMPANY, SKI/MEMORIAL shall retain and preserve one (1) copy of all records relating to the Study for two (2) years after the last marketing authorization for the Study Drug has been approved or COMPANY has discontinued its research with respect to the Study Drug and the FDA has been notified, or such longer period as shall be required by law (such period being referred to herein as the "Retention Period"). At the end of such period, SKI/MEMORIAL may destroy all such material upon giving COMPANY written notice of its intent to do so at least sixty (60) days prior to destruction.
9.2 Inventions. "Inventions" shall mean any invention that is conceived, developed and reduced to practice during or as a result of the performance of the Study. In the event that use of Actinium 225 and/or Lintuzurnab under this Agreement results in an Invention or discovery involving a new use, improvement, or enhancement of either or both of them, whether patentable or not, SKUMEMORIAL shall disclose the Invention to COMPANY. SKI/MEMORIAL shall grant COMPANY a royalty free license to its undivided interest in such Inventions for research, and development purposes only.. Any Inventions conceived or reduced to practice solely by SKI/MEMORIAL or its faculty, staff, employees, or students shall be the sole property of SKI/MEMORIAL. Inventions conceived and reduced to practice solely by COMPANY or its employees or subcontractors or agents shall be the sole property of COMPANY. Inventions conceived and reduced to practice jointly by SKI/MEMORIAL or its faculty, staff, employees, or students, together with one or more employees, subcontractors or agents of COMPANY, shall be owned jointly by SKI/MEMORIAL and COMPANY.
9.3 To the extent SKI may legally do so, SKI/MEMORIAL grants to COMPANY a right of first refusal to obtain an exclusive license to SKI's interest in any Inventions, through good faith negotiations and on commercially reasonable terms. The option shall extend for a period of six (6) months following disclosure of the Invention to the COMPANY. In the event the parties, acting in good faith, fail to reach a mutually acceptable agreement within six (6) months after commencing negotiations, SKI/MEMORIAL shall be entitled to negotiate a license with a third party for such patent applications.
9.4 Retention of Non-Exclusive License by SKUMEMORIAL. SKI/MEMORIAL shall retain an irrevocable, non-assignable, royalty free license to use for non-commercial research purposes any Inventions licensed to COMPANY pursuant to Section 9.3.
ARTICLE X - INDEMNIFICATION — INSURANCE
10.1 COMPANY shall indemnify, defend and hold SKI/MEMORIAL, and their affiliate corporation Memorial Sloan-Kettering Cancer Center harmless from and against all claims, causes of action, suits, damages and costs arising out of, resulting from, or otherwise in respect of, the manufacture and/or use of Actinium-225 or Lintuzumab — Actinium 225 by COMPANY'S staff or agents, except where such claims, causes of action, suits, damages and costs arc the result of COMPANY's use of the results of this study, noncompliance with the Protocol or are the result of gross negligence or willful misconduct by SKI/MEMORIAL, its investigators, staff, or agents_ COMPANY shall have no obligation to indemnify, defend or hold SKI/MEMORIAL and their affiliate corporation, Memorial Sloan Kettering Cancer Center, harmless from and against all claims, causes of action, suits, damages and costs arising directly from a failure by SKI/MEMORIAL, its staff or agents to: (i) comply with any applicable FDA or other governmental requirement; (ii) adhere to the terms of the Protocol. Furthermore, COMPANY shall indemnify, defend and hold SKI/MEMORIAL, and their affiliate corporation Memorial Sloan-Kettering Cancer Center harmless from and against all claims, causes of action, suits, damages and costs arising out of COMPANY'S use of the report or data of the Study.
10.2 SKI/MEMORIAL shall indemnify, defend or hold COMPANY harmless from and against all claims, causes of action, suits, damages and costs arising directly from a failure by SKI/MEMORIAL, its staff or agents to: (i) comply with any applicable FDA or other governmental requirement; (ii) adhere to the terms of the Protocol, or which are (iii) the result of gross negligence or willful misconduct by SKI/MEMORIAL, its investigators, staff, or agents, all except to the extent that such claims arise out of COMPANY'S gross negligence or willful misconduct.
10.3 As a condition to a party's right to indemnification hereunder, the claiming party must inform the other party of a claim as soon as is practical after it receives notice of the claim, permit the indemnifying party to control the defense of such claim, to select and engage counsel of its own choice to defend against such claims and to settle any claims or suits at its discretion, and otherwise cooperate fully with the indemnifying party in the defense of such claim. In no event shall the indemnifying party have any obligation hereunder with respect to claims or suits settled or compromised without its prior written consent.
ARTICLE XI — GENERAL
11.1 No right or license is granted under this Agreement by either party to the other either expressly or by implication, except those specifically set forth herein.
11.2 Unless otherwise specified in this Agreement, nothing contained in this Agreement shall impose an obligation of exclusivity on one party by the other. Both parties reserve the right to enter into and participate in other activities (either alone or with a third party) including, but not limited to, clinical trials and sponsored research projects.
11.3 All matters affecting the interpretation, validity and performance of this Agreement shall be governed by the laws of the State of New York applicable to agreements made and to be performed wholly within the State of New York. This Agreement, including the Protocol, sets forth the entire understanding between the parties herein, and cannot be changed or amended except by written agreement executed by the parties. In the event of any inconsistency in this Agreement, the inconsistency shall be resolved by giving precedence first, to the Articles of this Agreement, and then, to the Protocol. Notwithstanding the above, in the event of a conflict between the text of this Agreement and the text of the final, IRB-approved Protocol, the final Protocol shall control with respect to any matter for which the United States Food and Drug Administration (hereinafter "FDA") has promulgated regulations addressing the requirement set forth in the Protocol; this Agreement shall govern for all other matters. This Agreement may not be assigned by either party without the prior written consent of the other party.
11.4 All notices to be given by either party to the other shall be made in writing, delivered by any means providing proof of delivery, at the following addresses respectively:
SKI/MEMORIAL
Memorial Sloan-Kettering Cancer Center
1275 York Avenue
New York, New York 10065
(Attention: Director, Office of Technology Development
COMPANY
Actinium Pharmaceuticals
391 Lafayette Street
Newark, NJ 07105
(Attention: President and CEO)
Any notice shall be effective as of its date of receipt.
11.5 Except as set forth in Articles III and IV, as required by taw and/or as may be required in order to maintain a party's status as an exempt organization under Section 501 (c)(3) of the Internal Revenue Code and regulations thereunder, neither SKI/MEMORIAL nor COMPANY shall release any information, publicity, news releases or other public announcement, written or oral, with regard to the Agreement or any amendment thereto or to performance hereunder, to newspapers or any other mass communication media without the prior written approval of the other party, which approval will not be unreasonably withheld or delayed. COMPANY shall not use the name of SKUMEMORIAL and their affiliate corporation Memorial Sloan-Kettering Cancer Center, or a variant of any of the foregoing in any advertising, packaging or other promotional material in connection with the Study Drug except as may be required by law.
11.6 If any one or more of the provisions of this Agreement is held to be invalid or unenforceable from which no appeal can be or is taken, the provision shall be considered severed from this Agreement, and shall not serve to invalidate the remaining provisions hereof, so long as the essential benefits of this Agreement will still be realized. The parties shall make a good faith effort to replace the invalid or unenforceable provision with a valid one that, in its economic effect, is most consistent with the invalid or unenforceable provision.
IN WITNESS THEREOF, SKI/MEMORIAL and COMPANY have caused this Agreement to be executed in duplicate by their respective duly authorized officers.
| Actinium Pharmaceuticals |
|
|
SLOAN-KETTERING INSTITUTE FOR CANCER RESEARCH, AND
|
|
| |
|
|
|
|
|
| By: |
/s/Dragan Cicic
|
|
By: |
/s/ Eric Cottington
|
|
| |
Dragan Cicic
|
|
|
|
|
| |
CEO
|
|
|
Vice P esident, Research and Technology Management
|
|
|
Date: March 15, 2012
|
|
Date: March 27, 2012
|
|
| |
|
|
|
|
|
| |
|
|
By: |
/s/ George J. Bosl
|
|
| |
|
|
|
George J. Bosl, M.D.
|
|
| |
|
|
|
Chairman. Department of Medicine
|
|
| |
|
|
|
|
|
| |
|
|
By: |
/s/ Marcel R. M
|
|
| |
|
|
|
Marcel R. M. van den brink, M.D., PhD
|
|
| |
|
|
|
Head, Div. of hematology Oncology
|
|
| |
|
|
|
|
|
| |
|
|
By: |
/s/ Joseph Jurcic
|
|
| |
|
|
|
Joseph Jurcic, .M.D.
|
|
| |
|
|
|
Principal Investigator |
|
15